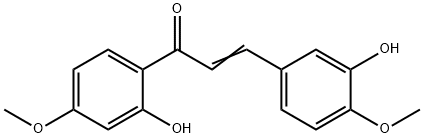
3,2'-Dihydroxy-4,4'-dimethoxychalcone synthesis
- Product Name:3,2'-Dihydroxy-4,4'-dimethoxychalcone
- CAS Number:2567-65-9
- Molecular formula:C17H16O5
- Molecular Weight:300.31
Yield:-
Reaction Conditions:
with potassium hydroxide in methanol at 20;Claisen-Schmidt Condensation;
Steps:
1.2. General synthetic procedures for thetarget compounds
General procedure: The strategy for the synthesis 3-Benzylchroman-4-onederivatives involved the preparation of2'-hydroxychalcone intermediate by Claisen-Schmidt condensation. Substitutedbenzaldehyde (leq) was reacted with2'-hydroxyacetophenone (leq) in 40-50% of MeOH/KOH. The mixture was poured into crushed ice acidified withdilute hydrochloric acid and stirred well. The reaction mixture was kept in therefrigerator for overnight to precipitate 2'-Hydroxychalcone. 2'-hydroxychalcone, saturated ammonium formate solution [methanol:THF (1:1)] and 10% Pd/C were refluxed for90 minutes. The reaction mixture was filtered.The product which remained in the filtrate wasisolated in good yield by dispersing the residue in water, extracting itwith ethyl acetate, and drying over anhydrous Na2SO4 to obtain2'-Hydroxydihydrochalcone. 2'-Hydroxydihydrochalcone was dissolved in ethanol and refluxedwith paraformaldehyde and 50% aqueous diethylamine for 9 hrs. Ethanol wasdistilled off, and the residue was takenup in ethyl acetate. Ethyl acetate was distilled off, and the oily residue was column chromatographed over silicausing pet ether: ethyl acetate (7:3) as eluent to get the targetcompounds 3a-3o in 60-70% yield.
References:
Simon, Lalitha;Abdul Salam, Abdul Ajees;Madan Kumar;Shilpa;Srinivasan;Byrappa [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 23,p. 5284 - 5290] Location in patent:supporting information

621-59-0
632 suppliers
$9.00/1g

2567-65-9
26 suppliers
inquiry
![Benzaldehyde, 4-methoxy-3-[(tetrahydro-2H-pyran-2-yl)oxy]-](/CAS/20210305/GIF/106852-87-3.gif)