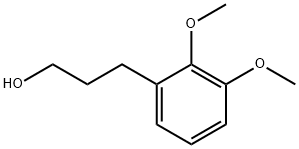
3-(2,3-DIMETHOXY-PHENYL)-PROPAN-1-OL synthesis
- Product Name:3-(2,3-DIMETHOXY-PHENYL)-PROPAN-1-OL
- CAS Number:103853-10-7
- Molecular formula:C11H16O3
- Molecular Weight:196.24
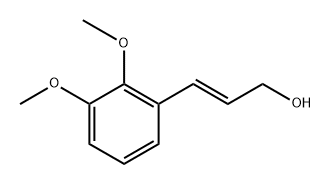
1137838-36-8
2 suppliers
inquiry

103853-10-7
8 suppliers
inquiry
Yield:103853-10-7 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol; for 1 h;
Steps:
3-(2,3-Dimethoxyphenyl)propan-1-ol (14).
To a solution of 13 (500 mg, 2.3 mmol) in MeOH (10 mL) was added in one portion Pd/C 10% (50 mg, 0.25 mmol). The reaction mixture was stirred for 1 h under hydrogen atmosphere at atmospheric pressure, filtered over celite and evaporated to dryness. The crude product 14 (colorless oil) was pure enough to be used in the next step. (497 mg, 2.5 mmol, 98 %, RF = 0.64, EtOAc/petroleum ether, 8:2). 1H-NMR (300 MHz, CDCl3): δ (ppm) = 1.81 (2H, qui, J = 6.7 Hz, -CH2-), 2.21 (1H, s, -OH), 2.71 (2H, t, J = 7.3 Hz, CH2-Ph), 3.55 (2H, t, J = 6.1 Hz, CH2-OH), 3.80 (3H, s, OMe), 3.83 (3H, s, OMe), 6.75 (2H, d, J = 8.1 Hz, aromatic H-4 and H-6), 6.97 (1H, dd, J = 7.5 Hz, 8.4 Hz, aromatic H-5). 13C-NMR (75.5 MHz, CDCl3): δ (ppm) = 25.7, 33.5, 55.7, 60.8, 61.6, 110.3, 122.1, 124.2, 135.4, 147.1, 152.6. HRMS (EI+) calculated m/z for C11H 16O3Na [M+Na]+ 219.099, found 219.100.
References:
Zingle, Catherine;Kuntz, Lionel;Tritsch, Denis;Grosdemange-Billiard, Catherine;Rohmer, Michel [Bioorganic and medicinal chemistry letters,2012,vol. 22,# 21,p. 6563 - 6567,5] Location in patent:supporting information Zinglé, Catherine;Kuntz, Lionel;Tritsch, Denis;Grosdemange-Billiard, Catherine;Rohmer, Michel [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 21,p. 6563 - 6567] Location in patent:supporting information
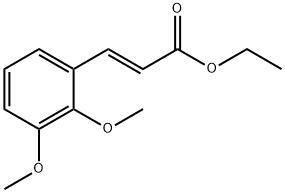
79618-90-9
3 suppliers
inquiry

103853-10-7
8 suppliers
inquiry
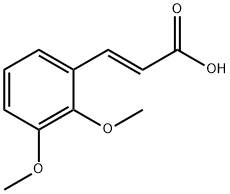
7345-82-6
127 suppliers
$10.00/5g

103853-10-7
8 suppliers
inquiry
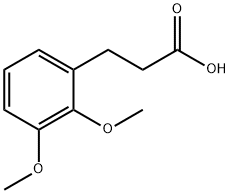
10538-48-4
74 suppliers
$11.00/250mg

103853-10-7
8 suppliers
inquiry

86-51-1
355 suppliers
$6.00/5g

103853-10-7
8 suppliers
inquiry