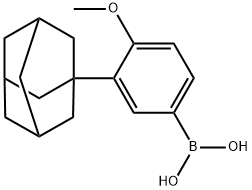
3-(1-ADAMANTYL)-4-METHOXYBENZENEBORONIC ACID synthesis
- Product Name:3-(1-ADAMANTYL)-4-METHOXYBENZENEBORONIC ACID
- CAS Number:459423-32-6
- Molecular formula:C17H23BO3
- Molecular Weight:286.17
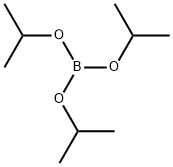
5419-55-6
379 suppliers
$12.00/5g

459423-32-6
91 suppliers
$50.00/25mg
Yield: 94.8%
Reaction Conditions:
Stage #1:2-(1-adamantyl)-4-bromoanisole with n-butyllithium in tetrahydrofuran at -75 - -70; for 1 h;
Stage #2:Triisopropyl borate in tetrahydrofuran at -70 - 20;
Stage #3: with hydrogenchloride;water in tetrahydrofuran at 20;
Steps:
1.a
Example 1 : EPO
References:
GALDERMA RESEARCH & DEVELOPMENT, S.N.C. WO2006/108717, 2006, A2 Location in patent:Page/Page column 8-9

104224-63-7
311 suppliers
$16.00/5g

459423-32-6
91 suppliers
$50.00/25mg

121-43-7
359 suppliers
$13.00/25mL

104224-63-7
311 suppliers
$16.00/5g

459423-32-6
91 suppliers
$50.00/25mg
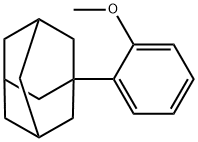
43109-77-9
62 suppliers
$100.00/50mg

121-43-7
359 suppliers
$13.00/25mL

104224-63-7
311 suppliers
$16.00/5g

459423-32-6
91 suppliers
$50.00/25mg