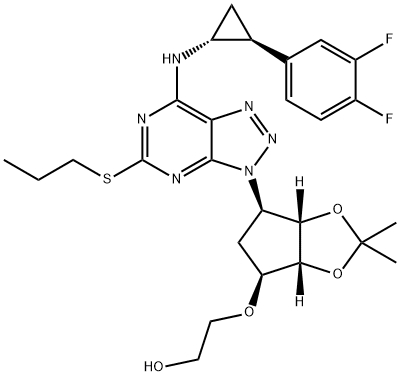
2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol synthesis
- Product Name:2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol
- CAS Number:274693-26-4
- Molecular formula:C26H32F2N6O4S
- Molecular Weight:562.63
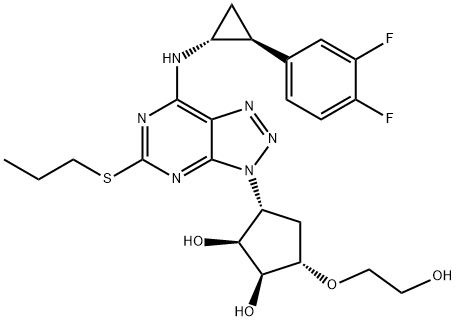
274693-27-5
640 suppliers
$13.00/10mg
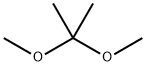
77-76-9
389 suppliers
$9.00/5ml
![2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol](/CAS/20150408/GIF/274693-26-4.gif)
274693-26-4
171 suppliers
inquiry
Yield:274693-26-4 93%
Reaction Conditions:
with toluene-4-sulfonic acid in acetone at 20 - 30; for 12 h;Inert atmosphere;
Steps:
1.1 step 1
Under an inert nitrogen atmosphere, washed 50mL three-necked round bottom flask was placed (1S, 2S, 3R, 5S) -3- (7 - [[(2S) -2- (3,4- difluorophenyl ) cyclopropyl] amino] -5- (mercapto propyl) -3H- [1,2,3] triazolo [4,5-d] pyrimidin-3-yl) -5- (2-hydroxyethoxy ) 2-diol (2g, 3.79mmol, 1.00 eq., 99%) and 4-methylbenzenesulfonate (20mg, 0.12mmol, 0.03 eq., 99%) in acetone (50 mL), then stirring at room temperature was added dropwise 2,2-dimethoxy propane (800mg, 7.68mmol, 2.03 eq).The resulting solution was stirred at 30 12 hours.Sodium bicarbonate pH-value of the solution was adjusted to 7.The mixture was filtered and the filtrate was concentrated in vacuo.The residue was purified by silica gel column with ethyl acetate: petroleum ether (1:50 to 1: 3) to afford 2g (93%) 2 - [[(3aR, 4S, 6R, 6aS) -6- (7 - [[(2S) -2- (3,4- difluorophenyl) cyclopropyl] amino] -5- (mercapto propyl) -3H- [1,2,3] triazolo [4, 5-d] pyrimidin-3-yl) -2,2-dimethyl - hexahydro-cyclopenta [d] [1,3] dioxol-4-yl] oxy] -1-ethyl - alcohol white oil.
References:
CN105481861,2016,A Location in patent:Paragraph 0053; 0054; 0055
![Carbamic acid, N-[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]-N-[5-(propylthio)-3-[(3aS,4R,6S,6aR)-tetrahydro-6-(2-hydroxyethoxy)-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]-3H-1,2,3-triazolo[4,5-d]pyrimidin-7-yl]-, 1,1-dimethylethyl ester](/CAS/20200515/GIF/1383715-61-4.gif)
1383715-61-4
0 suppliers
inquiry
![2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol](/CAS/20150408/GIF/274693-26-4.gif)
274693-26-4
171 suppliers
inquiry
![2-[[(3aR,4S,6R,6aS)-6-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]tetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-acetic acid methyl ester](/CAS/20150408/GIF/274693-25-3.gif)
274693-25-3
15 suppliers
inquiry
![2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol](/CAS/20150408/GIF/274693-26-4.gif)
274693-26-4
171 suppliers
inquiry