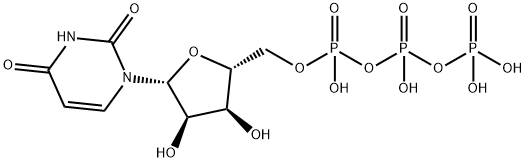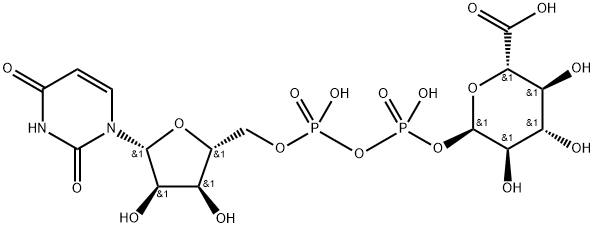
(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic synthesis
- Product Name:(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic
- CAS Number:2616-64-0
- Molecular formula:C15H22N2O18P2
- Molecular Weight:580.29
Yield:2616-64-0 89%
Reaction Conditions:
with glucuronokinase from Arabidopsis thaliana;pyrophosphatase from E. coli;UDP-sugar pyrophosphorylasegene from Arabidopsis thaliana;ATP;magnesium chloride in aq. buffer at 37; pH=8;Enzymatic reaction;
Steps:
1 4.6. One-pot three-enzyme synthesis of UDP-GlcA and UDP-GalA
General procedure: Preparative-scale synthesis of UDP-GlcA and UDP-GalA wereperformed in a 20 mL reaction system containing 50 mM pH 8.0TriseHCl buffer, 20 mM GlcA or GalA, 22 mM ATP, 22 mM UTP,20mMMgCl2, and 0.1e2mg of different enzymes. For the synthesisof UDP-GlcA, 0.6 mg of AtGlcAK, 0.4 mg of AtUSP, and 0.1 mg of EcPpA20 were employed; for the synthesis of UDP-GalA, 2 mg ofBiGalK, 0.4 mg of AtUSP, and 0.1 mg of EcPpA were employed. Thereaction mixtures were incubated at 37 C for 4e12 h with gentleshaking. The generation of UDP-sugars were monitored by TLC(Isopropanol:NH4OH:H2O7:3:2). After 90% of monosaccharidesconverted into UDP-sugar, reactions were quenched by boiling for5 min, centrifuged to remove precipitants, and concentrated forseparation. The separation of UDP-sugars from the reaction mixtureswere performed by gel filtration (Bio-Gel P2, Bio-Rad), fractionscontaining products were pooled and lyophilized. Digestion ofnucleotides by alkaline phosphatase and further P2 purificationwere applied to achieve minimum 95% purity. The final productswere lyophilized and characterized by HRMS and NMR.
References:
Guo, Yuxi;Fang, Junqiang;Li, Tiehai;Li, Xu;Ma, Cheng;Wang, Xuan;Wang, Peng G.;Li, Lei [Carbohydrate Research,2015,vol. 411,p. 1 - 5]

133-89-1
37 suppliers
inquiry
![(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic](/CAS/20210111/GIF/2616-64-0.gif)
2616-64-0
57 suppliers
inquiry

63-37-6
576 suppliers
$12.00/25g
![(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic](/CAS/20210111/GIF/2616-64-0.gif)
2616-64-0
57 suppliers
inquiry
58-97-9
314 suppliers
$9.00/100mg
![(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic](/CAS/20210111/GIF/2616-64-0.gif)
2616-64-0
57 suppliers
inquiry