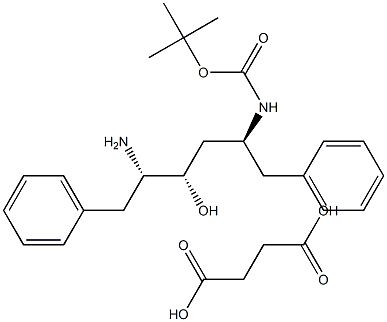
(2S,3S,5S)-5-tert-Butyloxycarbonylamino-2-amino-3-hydroxy-1,6-diphenylhexane succinate synthesis
- Product Name:(2S,3S,5S)-5-tert-Butyloxycarbonylamino-2-amino-3-hydroxy-1,6-diphenylhexane succinate
- CAS Number:256328-84-4
- Molecular formula:C27H38N2O7
- Molecular Weight:502.5998
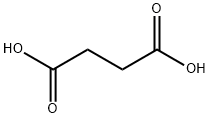
110-15-6
959 suppliers
$5.00/10g

256328-84-4
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexanewith ammonium formate;palladium 10% on activated carbon in methanol at 20; for 2 h;Heating / reflux;
Stage #2: succinic acid at 20 - 70; for 13 h;
Steps:
3
To the residue obtained from example 2 and ammonium formate (19.88g) in methanol (200 mL), was added 10% Palladium-Carbon (30.35 g, 50% wet) at room temperature. The mixture was stirred under reflux for 2 hours. The catalyst was filtered off and washed with methanol (100 mL). The residue obtained after removal of methanol under reduced pressure was taken up in methylene chloride (245 mL) and washed with aqueous sodium hydroxide (122.5 mL) then with sodium chloride solution (122.5 mL). The organic was concentrated to provide a thick oily mass. Isopropanol (588 mL) was added into the residue and stirred to yield a clear solution. Isopropanol (24.5 mL) was removed under reduced pressure at NMT 45°C. To the stirred above solution succinic acid (6.35 g) was added at 20-25°C. The mixture was slowly heated to 40-45°C to dissolve all succinic acid crystals and at about 70°C, a white solid started precipitated out. The mixture was kept stirring at 65-7O0C for 1 hour and then at 25-30°C for next 12 hours. The solid was filtered, washed with isopropanol (24.5 mL) and then dried under reduced pressure at 45-50°C for 12 hours to furnish the title compound as a white solid.Yield: 18.5 g
References:
WO2006/90264,2006,A1 Location in patent:Page/Page column 20
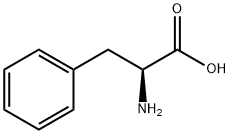
63-91-2
880 suppliers
$10.00/100G

256328-84-4
0 suppliers
inquiry