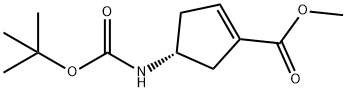
methyl (4R)-4-{[(tert-butoxy)carbonyl]amino}cyclopent-1-ene-1-carboxylate synthesis
- Product Name:methyl (4R)-4-{[(tert-butoxy)carbonyl]amino}cyclopent-1-ene-1-carboxylate
- CAS Number:251327-01-2
- Molecular formula:C12H19NO4
- Molecular Weight:241.28
![(1R-4S)-4-[[(1,1-dimethylethoxy)carbonyl]amino]- 2-Cyclopentene-1-carboxylic acid methyl ester](/CAS2/GIF/251326-99-5.gif)
251326-99-5
40 suppliers
inquiry
![methyl (4R)-4-{[(tert-butoxy)carbonyl]amino}cyclopent-1-ene-1-carboxylate](/CAS/20180713/GIF/251327-01-2.gif)
251327-01-2
13 suppliers
inquiry
Yield:251327-01-2 93%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 20; for 66 h;
Steps:
4.4. (R)-Methyl 4-(tert-butoxycarbonylamino)cyclopent-1-enecarboxylate 2
To a 20-L rotary evaporator flask was charged 7 (368.3 g, 1.53 mol), dichloromethane (2.7 L), and DBU (325 mL, 2.14 mol) at rt and the flask swirled for 66 h. The flask was chilled in an ice-bath, poured into a 22-L separatory funnel containing ice-cold aqueous HCl (0.5 M, 4.2 L) and the contents were gently swirled (to prevent emulsification). The layers were separated and the aqueous layer was back-extracted with dichloromethane (300 mL). The combined organic layers were washed with water (600 mL), dried over Na2SO4, filtered, and concentrated under reduced vacuum. The crude product was recrystallized from heptane (900 mL) in a round bottomed flask and heated until all of the solid dissolved. The heat was turned off and the flask swirled as it was allowed to return to rt. The product precipitated as yellow prills, and was collected by filtration and washed with heptane (2 x 150 mL). The collected solid was dried in a vacuum oven (26 °C, 30 in Hg) and provided 2 (341 g) in 93% isolated yield. Mp 88.5 °C. 1H NMR (400 MHz, CDCl3) δ 6.69-6.74 (2H, m), 4.74 (1H,br s), 4.36 (1H, br s), 3.73 (3H, s), 2.86-3.01 (1H, m), 2.34-2.48 (1H, m), 1.44 (9H, s). 13C NMR (100 MHz, CDCl3) δ 165.11, 155.51, 141.20, 134.43, 79.5, 51.57, 50.2, 41.18, 39.17, 28.42. [α]25D = -10.28 (c 1.13, MeOH). Anal. Calcd for C12H19NO4 C 59.73,H 7.94, N 5.81. Found: C 59.97, H 8.04, N 6.03.
References:
Cai, Chaozhong;Kang, Fu-An;Beauchamp, Derek A.;Sui, Zhihua;Russell, Ronald K.;Teleha, Christopher A. [Tetrahedron Asymmetry,2013,vol. 24,# 11,p. 651 - 656]
![(1S,4R)-2-Aza-bicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/130931-83-8.gif)
130931-83-8
168 suppliers
$8.00/100mg
![methyl (4R)-4-{[(tert-butoxy)carbonyl]amino}cyclopent-1-ene-1-carboxylate](/CAS/20180713/GIF/251327-01-2.gif)
251327-01-2
13 suppliers
inquiry
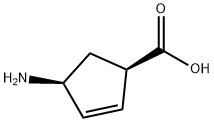
134003-04-6
103 suppliers
$82.00/100mg
![methyl (4R)-4-{[(tert-butoxy)carbonyl]amino}cyclopent-1-ene-1-carboxylate](/CAS/20180713/GIF/251327-01-2.gif)
251327-01-2
13 suppliers
inquiry
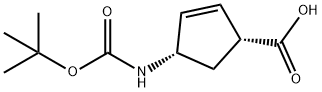
151907-80-1
161 suppliers
$22.00/250mg
![methyl (4R)-4-{[(tert-butoxy)carbonyl]amino}cyclopent-1-ene-1-carboxylate](/CAS/20180713/GIF/251327-01-2.gif)
251327-01-2
13 suppliers
inquiry