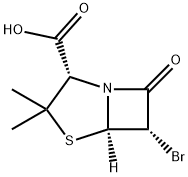
[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid synthesis
- Product Name:[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
- CAS Number:24138-28-1
- Molecular formula:C8H10BrNO3S
- Molecular Weight:280.14
![(2S,5R,6S)-6-Amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/20210305/GIF/21794-93-4.gif)
21794-93-4
1 suppliers
inquiry
![[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/GIF/24138-28-1.gif)
24138-28-1
29 suppliers
inquiry
Yield: 90%
Reaction Conditions:
with sulfuric acid;potassium bromide in ethanol at 4 - 8;
Steps:
1.1
Step 1. Production of 6α-Bromopenicillanic acid (BPA) (compound II) 2.5 L of 1.24 molar sulphuric acid (3.125 mol) was stirred at 4°C in a 6 L flask. 218.4 g (1.0 mol) of 6-APA (99%) (compound I) following 601 g (5.05 mol) of potassium bromide and 2000 mL of ethanol were added, maintaining the temperature between 4 to 8°C. Inorganic salts were removed by filtration. The resulting cake was washed by 2 x 1.25 L of cooled dichloromethane. The aqueous phase was extracted twice using the previous washing liquor and 3 x 500 mL of cooled dichloromethane. The organic phases were combined (approx. 4.0 L) and washed with 2 x 200 mL of 30% brine at 4°C. The greenish-brown solution was concentrated to 700 mL in vacuum. The precipitate was removed by filtration and the solution was kept below 0°C and used without further purification in the next reaction step. Yield: 90% (by titration)
References:
Helm AG EP1686131, 2006, A2 Location in patent:Page/Page column 7

551-16-6
473 suppliers
$7.00/10g
![[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/GIF/24138-28-1.gif)
24138-28-1
29 suppliers
inquiry
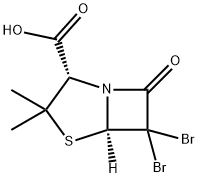
24158-88-1
70 suppliers
$500.08/5MG
![[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/GIF/24138-28-1.gif)
24138-28-1
29 suppliers
inquiry

24158-88-1
70 suppliers
$500.08/5MG
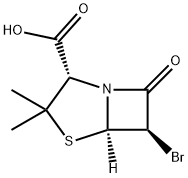
26631-90-3
54 suppliers
inquiry
![[2S-(2alpha,5alpha,6alpha)]-6-bromo-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/GIF/24138-28-1.gif)
24138-28-1
29 suppliers
inquiry
![(2S-cis)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid](/CAS/GIF/87-53-6.gif)
87-53-6
15 suppliers
inquiry