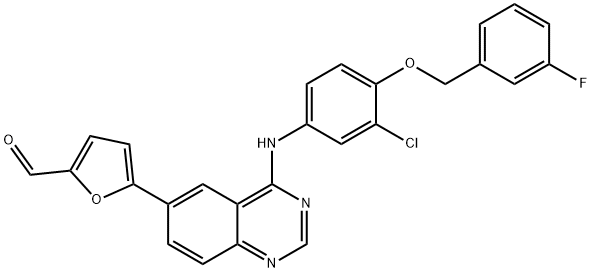
5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde synthesis
- Product Name:5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde
- CAS Number:231278-84-5
- Molecular formula:C26H17ClFN3O3
- Molecular Weight:473.88
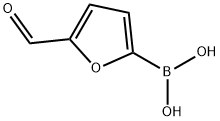
27329-70-0
466 suppliers
$6.00/1g
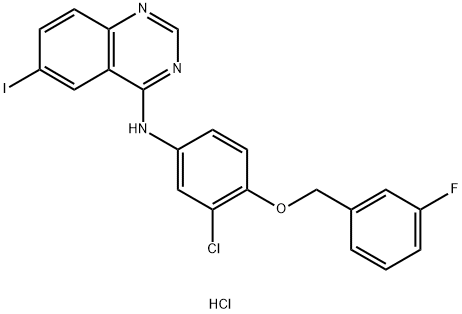
![N-[3-Chloro-4-(3-fluorobenzyloxy)phenyl]-6-iodoquinazolin-4-amine](/CAS/GIF/231278-20-9.gif)
231278-20-9
299 suppliers
$10.00/250mg
![5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde](/CAS/GIF/231278-84-5.gif)
231278-84-5
286 suppliers
$8.00/250mg
Yield:231278-84-5 98.7%
Reaction Conditions:
with potassium carbonate;palladium diacetate in tetrahydrofuran;ethanol for 0.666667 h;Product distribution / selectivity;Reflux;
Steps:
13
Example 13: preparation of crystalline lapatinib aldehyde base[00098] To a 3L reactor lOOg of compound of formula C, 37.35g of 5-fo?nyl-2- furanboronic acid, 1.33g of palladium acetate, 54.66g of potassium carbonate, 750 ml of absolute ethanol and 750ml of THF were added. The suspension was stirred and heated to reflux (T(jacket) = 75°C) for 40 minutes. The reaction mixture was cooled to room temperature (T(jacket) = 200C) and diluted with 750 ml of THF and 750 ml of absolute ethanol. The resulting mixture was stirred at 250C for an hour. Inorganic salts were filtered off in vacuum, washed with 100 ml of absolute ethanol, 100 ml of THF and discarded. The filtrate combined with washings was transferred into a 1OL reactor equipped with a mechanical stirrer and a dropping funnel. 3L of water was added dropwise into the solution of lapatinib-aldehyde base in EtOH/THF (1 :1) for an hour (T(jacket) = 200C). The resulting yellow suspension was stirred at RT (T(jacket) = 200C) for 1.5 hour. The yellow solid was filtered in vacuum and washed over the filter with 100 ml of cold absolute ethanol. It was allowed to dry in a vacuum oven at 400C for 16 hours, and additional 24 hours in vacuum oven at 600C to give 92.56g of final product (Yield - 98.7%; Purity - 99.12%)
References:
TEVA PHARMACEUTICAL INDUSTRIES LTD.;TEVA PHARMACEUTICALS USA, INC.;METSGER, Leonid;YURKOVSKI, Slavik;GOROHOVSKY-ROSENBERG, Sofia;KIPNIS, Noa;LAVY, Dikla WO2010/17387, 2010, A2 Location in patent:Page/Page column 24
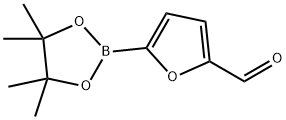
851684-46-3
6 suppliers
$45.00/10mg

27329-70-0
466 suppliers
$6.00/1g
![5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde](/CAS/GIF/231278-84-5.gif)
231278-84-5
286 suppliers
$8.00/250mg

27329-70-0
466 suppliers
$6.00/1g
![6-Bromo-N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]quinazolin-4-amine](/CAS2/GIF/944549-41-1.gif)
944549-41-1
17 suppliers
inquiry
![5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde](/CAS/GIF/231278-84-5.gif)
231278-84-5
286 suppliers
$8.00/250mg
273731-82-1
44 suppliers
$38.00/250mg
![N-[3-Chloro-4-(3-fluorobenzyloxy)phenyl]-6-iodoquinazolin-4-amine](/CAS/GIF/231278-20-9.gif)
231278-20-9
299 suppliers
$10.00/250mg
![5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde](/CAS/GIF/231278-84-5.gif)
231278-84-5
286 suppliers
$8.00/250mg
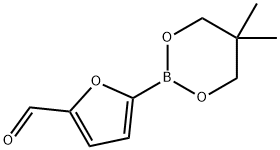
1218791-07-1
34 suppliers
$50.00/100mg
![N-[3-Chloro-4-(3-fluorobenzyloxy)phenyl]-6-iodoquinazolin-4-amine](/CAS/GIF/231278-20-9.gif)
231278-20-9
299 suppliers
$10.00/250mg
![5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde](/CAS/GIF/231278-84-5.gif)
231278-84-5
286 suppliers
$8.00/250mg
