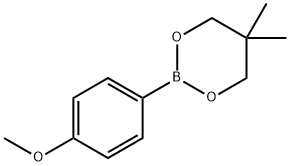
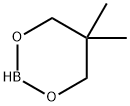
2-(4-Methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborinane synthesis
- Product Name:2-(4-Methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborinane
- CAS Number:213596-33-9
- Molecular formula:C12H17BO3
- Molecular Weight:220.07

696-62-8
349 suppliers
$5.00/1g
126-30-7
623 suppliers
$8.00/100g

213596-33-9
31 suppliers
$79.00/1g
Yield:213596-33-9 99%
Reaction Conditions:
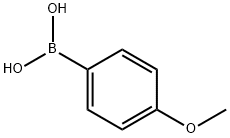
Stage #1:para-iodoanisole with diisopropopylaminoborane;triethylamine;triphenylphosphine;palladium dichloride in tetrahydrofuran at 65; for 12 h;Inert atmosphere;Alcaraz-Vaultier borylation;
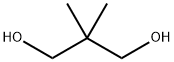
Stage #2:2,2-Dimethyl-1,3-propanediol in tetrahydrofuran at 0 - 25; for 0.25 h;Inert atmosphere;Further stages;
Steps:
4.3. General procedure for the synthesis of an aryl boronic ester from the reaction of aryl iodides or bromides with BH2N(iPr)2 in the presence of a palladium catalyst
General procedure: Triphenylphosphine (0.131 g, 0.5 mmol, 20 mol %), p-iodoanisol (0.585 g, 2.5 mmol), and triethylamine (1.78 mL, 12.5 mmol) were added to a 50 mL round-bottomed flask equipped with a sidearm, condenser, and stir bar. This solution was then degassed by alternating vacuum and argon three times. Palladium dichloride (0.023 g, 0.13 mmol, 5 mol %) was then added under positive argon pressure. After stirring at room temperature for 15 min diisopropylaminoborane (5 mL, 1 M THF, 5 mmol) was added and the reaction mixture was degassed again by alternating vacuum and argon three times. The reaction solution was then heated to reflux. After 12 h of reflux the reaction was cooled to 0 °C and neopentyl glycol (6 mL, 1 M solution in THF, 6 mmol) was slowly added through the condenser. After warming to 25 °C and stirring for 15 min. 1 M HCl (6 mL) was added and the mixture was filtered through a pad of Celite. The mixture was then extracted with diethyl ether (3×10 mL), combining all the organic fractions together. The organic fractions were then washed with a brine solution (2×10 mL), dried with magnesium sulfate and filtered. The solvent was removed under reduced pressure yielding 2-(4-methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborinane as a yellow oil.
References:
Haddenham, Dustin;Bailey, Christopher L.;Vu, Chau;Nepomuceno, Gabby;Eagon, Scott;Pasumansky, Lubov;Singaram, Bakthan [Tetrahedron,2011,vol. 67,# 3,p. 576 - 583] Location in patent:experimental part
5720-07-0
466 suppliers
$6.00/5g

126-30-7
623 suppliers
$8.00/100g

213596-33-9
31 suppliers
$79.00/1g

104-92-7
428 suppliers
$10.00/10g
668987-38-0
0 suppliers
inquiry

213596-33-9
31 suppliers
$79.00/1g

696-62-8
349 suppliers
$5.00/1g
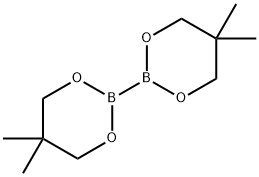
201733-56-4
261 suppliers
$5.00/1g

213596-33-9
31 suppliers
$79.00/1g