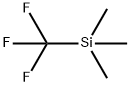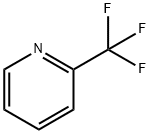
2-(Trifluoromethyl)pyridine synthesis
- Product Name:2-(Trifluoromethyl)pyridine
- CAS Number:368-48-9
- Molecular formula:C6H4F3N
- Molecular Weight:147.1
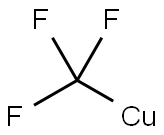
109-04-6
531 suppliers
$6.00/25g
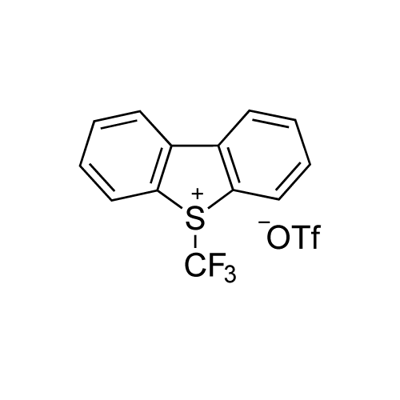
129946-88-9
145 suppliers
$12.00/100mg

368-48-9
220 suppliers
$8.00/1g
Yield:368-48-9 96%
Reaction Conditions:
with copper in N,N-dimethyl-formamide at 0 - 80; for 4 h;Inert atmosphere;
Steps:
6 Example 6: Preparation of 2-trifluoromethylpyridine
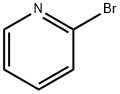
2-bromopyridine (1.58 g, 0.01 mol) was added to a 100 ml three-necked flask under N2 protection.25 ml of DMF, Cu powder (1.92 g, 0.03 mol), stirring was started, and the ice water bath was cooled to 0-5 °C. add Umemoto reagent (8.76g, 0.02mol).After stirring for 1 h in an ice water bath, the mixture was further heated to 80 ° C for 3 h.The reaction solution was subjected to 19F NMR analysis using OTf as an internal standard, and the yield was 96%.
References:
CN108239021,2018,A Location in patent:Paragraph 0060-0062