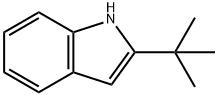
2-TERT-BUTYL-1H-INDOLE synthesis
- Product Name:2-TERT-BUTYL-1H-INDOLE
- CAS Number:1805-65-8
- Molecular formula:C12H15N
- Molecular Weight:173.25
61495-04-3
56 suppliers
$57.00/1g

1805-65-8
87 suppliers
$22.00/100mg
Yield: 95%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane at 20;Inert atmosphere;
Steps:
8 Example 8. Synthesis of 2-tert-butyl-1 H-lndole.
Example 8. Synthesis of 2-tert-butyl-1 H-lndole. In a one L flask, a solution of N-o-Tolylpivalamide (35g, 0.183 mol, 1 eq) (obtained in Example 7) in dry THF (120 mL) was made under inert atmosphere. After cooling in a water bath, n- butyllithium (2.5 M in hexane, 220 mL, 0.55 mol, 3 eq) was added dropwise. The reaction mixture was stirred overnight at room temperature, after which it was cooled in an ice bath. Once the solution was cooled, saturated aqueous ammonium chloride solution (340 mL) was added slowly. The water phase was extracted with ethyl acetate (340 mL), after which the combined organic phases were dried on magnesium sulfate and concentrated in vacuo, to afford a brown solid (95%). MW.: 173.25 g/mol. 1H-NMR (500 MHz, CDCI3): δ (ppm) = 1 .32 (s, 9H, C(CH3)3), 6.18 (d, 1 H, t-Bu C-CH), 7.02 (m, 2H, ArH), 7.24 (d, 1 H, ArH), 7.46 (d, 1 H, ArH), 7.87 (br. s, 1 H, NH). 13C-NMR (125 MHz, CDCI3): δ (ppm) = 30.33 (CH3), 31 .84 (C), 96.96 (CH), 1 10.34 (CH), 1 19.60 (CH), 1 19.98 (CH), 121 .07 (CH), 128.51 (C), 135.76 (C), 148.77 (C).
References:
UNIVERSITEIT GENT;DU PREZ, Filip;WINNE, Johan;BILLIET, Stijn;DE BRUYCKER, Kevin WO2015/18928, 2015, A1 Location in patent:Page/Page column 151-152
630-18-2
188 suppliers
$10.00/1g

95-52-3
300 suppliers
$10.00/5 g

1805-65-8
87 suppliers
$22.00/100mg

75-97-8
238 suppliers
$10.00/1g
59-88-1
361 suppliers
$15.00/10g

1805-65-8
87 suppliers
$22.00/100mg
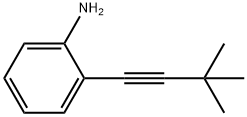
116491-51-1
1 suppliers
inquiry

1805-65-8
87 suppliers
$22.00/100mg
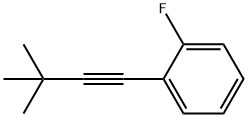
1436919-25-3
1 suppliers
inquiry

1805-65-8
87 suppliers
$22.00/100mg