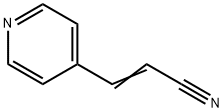
2-Propenenitrile,3-(4-pyridinyl)-(9CI) synthesis
- Product Name:2-Propenenitrile,3-(4-pyridinyl)-(9CI)
- CAS Number:24490-79-7
- Molecular formula:C8H6N2
- Molecular Weight:130.15

1121-60-4
508 suppliers
$10.60/10gm:

24490-79-7
10 suppliers
inquiry
Yield:24490-79-7 77%
Reaction Conditions:
Stage #1: diethyl 1-cyanomethylphosphonatewith sodium hydride in tetrahydrofuran; for 1 h;
Stage #2: pyridine-2-carbaldehyde in tetrahydrofuran at 0 - 20; for 3 h;
Steps:
148 Reference Example 148
Reference Example 148 3-(4-Pyridyl)acrylonitrile sodium hydride (ca. 60 %, 1.0 g, 28.1 mmol) was dissolved in tetrahydrofuran (10 ml), and a solution of diethyl cyanomethylphosphonate (4.7 g, 26.8 mmol) in tetrahydrofuran (5 ml) was added under ice cooling condition.. The mixture was stirred for 1 hr.. Successively, a solution of 2-pyridinecarbaldehyde (2.7 g, 25.0 mmol) in tetrahydrofuran (5 ml) was added to the mixture, and the mixture was stirred at 0 °C to room temperature for 3 hrs.. The reaction mixture was combined with water under ice cooling condition, concentrated under reduced pressure to the half amount and extracted with ethyl acetate.. The organic layer was washed with saturated brine and dried over magnesium sulfate.. The solvent was evaporated, and the residue was subjected to a silica gel (100 g) column chromatography.. The fractions eluted with hexane-ethyl acetate (2:1, v/v) were collected and concentrated to give the titled compound (a mixture of E-form and Z-form, 2.5 g, 77 %).1H-NMR (CDCl3) δ: 5.70 (0.18H, d, J = 12.1 Hz), 6.10 (0.82H, d, J = 16.7 Hz), 7.12 (0.18H, d, J = 12.1 Hz), 7.29 (0.82H, d, J = 16.7 Hz), 7.30-7.39 (1.6H, m), 7.63 (0.4H, d, J = 6.2 Hz), 8.70 (1.6H, d, J = 6.2 Hz), 8.75 (0.4H, d, J = 6.2 Hz).
References:
EP1424336,2004,A1 Location in patent:Page 245-246
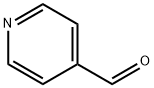
872-85-5
482 suppliers
$10.00/1g

24490-79-7
10 suppliers
inquiry