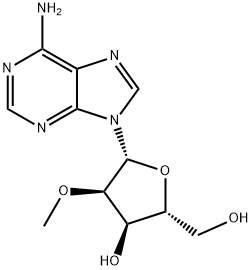
2'-O-Methyladenosine synthesis
- Product Name:2'-O-Methyladenosine
- CAS Number:2140-79-6
- Molecular formula:C11H15N5O4
- Molecular Weight:281.27
Methylation of adenosine with methyl iodide in anhydrous alkaline medium
Adenosine is treated with CH3I in an anhydrous alkaline medium at 0°C for 4 h. The major products of this reaction are monomethylated adenosine at either the 2′-O or 3′-O position (total of 64%) and the side products are dimethylated adenosine (2′,3′-O-dimethyladenosi, 21%, and N6-2′-O-dimethyladenosine, 11%). The ratio of 2′-O- and 3′-O-methyladenosine has been found to be 8 to 1. Therefore, this reaction preferentially favors the synthesis of 2′-O-methyladenosine. The monomethylated adenosine is isolated from reaction mixture by a silica gel column chromatography. Then the pure 2′-O-methyladenosine can be separated by crystallization in ethanol from the mixture of 2′-O and 3′-O-methylated isomers. The overall yield of 2′-O-methyladenosine is 42%.
https://doi.org/10.1016/0304-4165(80)90276-7
194034-61-2
6 suppliers
inquiry

2140-79-6
239 suppliers
$14.00/10mg
Yield:2140-79-6 80%
Steps:
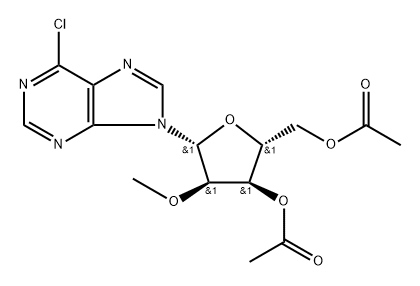
7 Synthesis of 2'-O-Methyl-Guanosine and Adenosine from 2-amino-6-chloropurine riboside (Scheme 2; FIG. 8).
Subsequent amination of (7) with methanolic ammonia at 125° C. for 4 hours yields 2'-O-Methyl-adenosine (8) in 80% yield.
References:
Ribozyme Pharmaceuticals, Inc. US5962675, 1999, A
57817-83-1
29 suppliers
inquiry

2140-79-6
239 suppliers
$14.00/10mg
17287-03-5
68 suppliers
$75.00/5mL
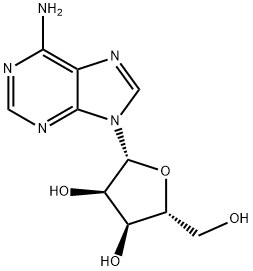
58-61-7
870 suppliers
$9.00/1g

2140-79-6
239 suppliers
$14.00/10mg
17287-03-5
68 suppliers
$75.00/5mL

58-61-7
870 suppliers
$9.00/1g
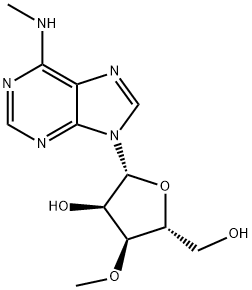
60037-52-7
4 suppliers
inquiry

57817-83-1
29 suppliers
inquiry

2140-79-6
239 suppliers
$14.00/10mg

10300-22-8
85 suppliers
$72.00/100mg
