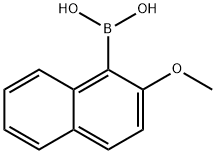
(2-METHOXY-1-NAPHTHYL)BORONIC ACID synthesis
- Product Name:(2-METHOXY-1-NAPHTHYL)BORONIC ACID
- CAS Number:104116-17-8
- Molecular formula:C11H11BO3
- Molecular Weight:202.01
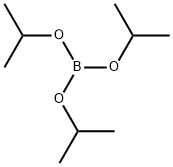
5419-55-6
373 suppliers
$12.00/5g
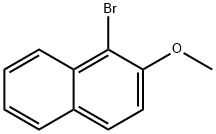
3401-47-6
167 suppliers
$59.00/5G

104116-17-8
133 suppliers
$65.00/1g
Yield:104116-17-8 86%
Reaction Conditions:
Stage #1: 1-bromo-2-methoxynaphthalenewith magnesium in tetrahydrofuran at 20; for 1 h;Heating;
Stage #2: Triisopropyl borate in tetrahydrofuran at -78 - 20; for 18 h;
Stage #3: with water in tetrahydrofuran; for 1 h;
Steps:
3.2.1 Method 1
Magnesium (5.00g, 0.21mol) was activated by heating and stirring in vacuo for 1h at 80°C after which time it was allowed to cool to room temperature. Dry THF (20mL) was added under an atmosphere of nitrogen. A solution of 1-bromo-2-methoxynaphthalene (10.9g, 46.3mmol) in THF was then added slowly via cannula to give a black suspension. The mixture was heated with a heat gun at regular intervals during the addition period. The solution was then allowed to stir for 1h at room temperature. The resulting mixture was added slowly via cannula to a solution of triisopropylborate (14.6mL, 63.3mmol) in THF (20mL) at -78°C. The reaction mixture was allowed to warm to room temperature while stirring overnight (18h). Water (40mL) was added and the suspension was allowed stir for a further 1h. The reaction mixture was evaporated in vacuo and dichloromethane (100mL) was added. The organic layer was separated, and the aqueous layer was washed with dichloromethane (4×50mL). The combined organic extracts were dried over MgSO4 and filtered. The solvent was removed in vacuo to give a brown solid, which was stirred in pentane overnight producing the title compound 10 (8.04g, 86%) as a white powder. m.p. 116-118°C (lit [45]. m.p. 117-119°C); 1H NMR (300MHz; DMSO-d6) δ=8.27 (s, 2H, B(OH)2), 7.89-7.81 (m, 2H), 7.69 (d, 1H, J=8.3Hz), 7.44-7.28 (m, 3H), and 3.85 (s, OCH3); 13C NMR (75MHz; DMSO-d6) 159.0 (4°), 136.2 (4°), 129.8, 128.9 (4°), 128.4, 127.8 (2C), 126.3, 123.5, 114.1, 56.6 (OMe); IR (KBr) νmax 3350, 1589, 1512, 1333, 1244, and 1063cm-1.
References:
Sweetman, Brian A.;Guiry, Patrick J. [Tetrahedron,2018,vol. 74,# 38,p. 5567 - 5581]

121-43-7
353 suppliers
$14.00/25mL

3401-47-6
167 suppliers
$59.00/5G

104116-17-8
133 suppliers
$65.00/1g

3401-47-6
167 suppliers
$59.00/5G

104116-17-8
133 suppliers
$65.00/1g
688-74-4
324 suppliers
$10.40/25mL

3401-47-6
167 suppliers
$59.00/5G

104116-17-8
133 suppliers
$65.00/1g
573-97-7
243 suppliers
$5.00/1g

104116-17-8
133 suppliers
$65.00/1g
