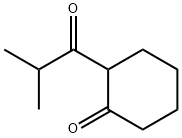
2-Isobutyrylcyclohexanone synthesis
- Product Name:2-Isobutyrylcyclohexanone
- CAS Number:39207-65-3
- Molecular formula:C10H16O2
- Molecular Weight:168.23

108-94-1
543 suppliers
$12.00/50g

79-30-1
367 suppliers
$10.00/10g

39207-65-3
109 suppliers
$24.00/1g
Yield:-
Reaction Conditions:
Stage #1:cyclohexanone with lithium hexamethyldisilazane in tert-butyl methyl ether;toluene at 0; for 0.0333333 h;Inert atmosphere;
Stage #2:isobutyryl chloride in tert-butyl methyl ether;toluene at 0; for 0.0333333 h;
Steps:
4.J
Example 4. Preparation of 3-hydroxy-l-isopropyl-5,6,7,8-tetrahydroisoquinoline-4- carbonitrile Intermediates.3-hydroxy- 1 -isopropyl-5,6,7,8-tetrahydroisoquinoline-4-carbonitrile intermediates were prepared according to Scheme 3. These intermediate were also used as a substitute for 8-cyclopropyl-6- hydroxy-3,3-dimethyl-3,4-dihydro-lH-pyrano[3,4-c]pyridine-5-carbonitrile (3) in Scheme 1 to produce additional compounds of the invention.S heme 3:Step J: 2-isobutyrylcyclohexanone (12; R=R=H). A 250 mL three-neck round bottom flask equipped with a stirring bar was charged with cyclohexanone (4.91 g, 50 mmol) and 87 mL of dry toluene. The solution was purged with nitrogen and cooled to 0°C. With stirring, a solution of LiHMDS (1.0M soln. in methyl tert-butyl ether, 52.5 mL, 52.5 mmol) was added dropwise, and the reaction mixture was allowed to stir for 2 min at 0°C before isobutyryl chloride (2.66 g, 25 mmol) was added with vigorous stirring. After an additional 2 min at 0°C, the cold bath was removed and after 5 min, the reaction mixture was quenched with acetic acid (20 mL, 50% AcOH/H20). After partitioning between H20 and ether, the organic layer was washed with brine, dried over anhy. Na2SC>4 and concentrated in vacuo. Flash column chromatography (30 % ethyl acetate/petroleum ether) afforded 4.4 g of title compound as yellowish oil. MS (ES) M+H expected 169.1 , found 169.1. .H NMR (DMSO-dg) δ 16.36 (s, 1H), 2.85 - 2.96 (m, 1H), 2.38 (qd, J = 6.4, 1.1 Hz, 4H), 1.69 - 1.73 (m, 4H), 1.13 (s, 3H), 1.11 (s, 3H).
References:
AGIOS PHARMACEUTICALS, INC.;CAO, Sheldon;POPOVICI-MULLER, Janeta;SALITURO, Francesco G.;SAUNDERS, Jeffrey;TAN, Xuefei;TRAVINS, Jeremy;YAN, Shunqi;YE, Zhixiong WO2012/171506, 2012, A1 Location in patent:Page/Page column 110
547-63-7
228 suppliers
$13.00/5g

108-94-1
543 suppliers
$12.00/50g

39207-65-3
109 suppliers
$24.00/1g
670-80-4
146 suppliers
$19.00/10 g

79-30-1
367 suppliers
$10.00/10g

39207-65-3
109 suppliers
$24.00/1g