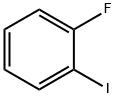
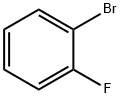
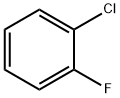
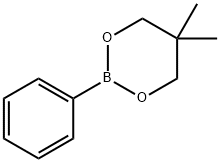
2-Fluorobiphenyl synthesis
- Product Name:2-Fluorobiphenyl
- CAS Number:321-60-8
- Molecular formula:C12H9F
- Molecular Weight:172.2
Yield:321-60-8 97%
Reaction Conditions:
with C28H40Br4N4Pd2;potassium carbonate in water;acetone at 20; for 1 h;Suzuki Coupling;
Steps:
4.3 General procedure of Suzuki reaction
General procedure: A mixture of aryl halide (1 mmol), arylboronic acid (1.2 mmol), catalyst A (1 mol %, 0.0096 g), K2CO3 (2 mmol), and (1:1) acetone/water mixed solvent (3 mL) were taken in 25 mL round bottom flask and the mixture was stirred at room temperature (40 °C for heteroaryl halides) until the completion of reaction (required time given in Tables 3-5). The reaction mixture was then diluted with water (20 mL) and extracted three times with dichloromethane (3×10 mL). The combined organic layer was washed with brine (20 mL) and dried over anhydrous Na2SO4. After that it was concentrated under reduced pressure and the crude product was purified by column chromatography on silica gel (60-120 mesh) using petroleum ether (60-80 °C) and ethyl acetate were as the eluent.
References:
Gupta, Sumanta;Basu, Basudeb;Das, Sajal [Tetrahedron,2013,vol. 69,# 1,p. 122 - 128]
![Methanesulfonic acid, 1,1,1-trifluoro-, [1,1'-biphenyl]-2-yl ester](/CAS/20200611/GIF/17763-65-4.gif)
17763-65-4
2 suppliers
inquiry

321-60-8
280 suppliers
$9.00/1g