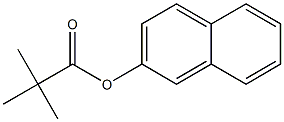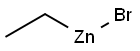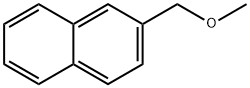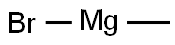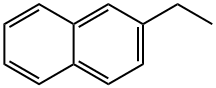
2-ETHYLNAPHTHALENE synthesis
- Product Name:2-ETHYLNAPHTHALENE
- CAS Number:939-27-5
- Molecular formula:C12H12
- Molecular Weight:156.22
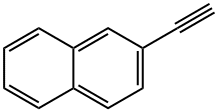
2949-26-0
108 suppliers
$24.00/250mg

939-27-5
93 suppliers
$16.00/1g
Yield:939-27-5 77%
Reaction Conditions:
with (R)-(-)-5,5'-bis[di(3,5-ditert-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole;methyl dimethoxy silane;copper (II) acetate in tetrahydrofuran;isopropanol at 60; for 14 h;Glovebox;
Steps:
1; 2 General procedure for transfer hydrogenation (C).
General procedure: In a N2 filled glovebox, (R or S)-DTBM-SEGPHOS (10.4 mg, 0.0088 mmol, 0.022 eq.), Cu(OAc)2 (40 μL of a 0.2 M solution in THF, 0.008 mmol, 0.02 eq.), and THF (0.16 mL) were added to an oven-dried 2- dram vial followed by dropwise addition of dimethoxy(methyl)silane (247 μL, 2 mmol, 5 eq.). A color change from green/blue to orange was observed while stirring for 15 minutes. In a separate oven-dried 1-dram vial was added the alkyne substrate (0.4 mmol, 1 eq.), THF (0.2 mL), and either ethanol or 2-propanol (2.4-5 eq. based on substrate). The solution in the 1- dram vial was added dropwise over 20 seconds to the 2-dram vial. The total volume of THF was calculated based on having a final reaction concentration of 1M based on the alkyne substrate. The 2-dram vial was capped with a red pressure relief cap, taken out of the glovebox, and stirred for 9-24 h at the appropriate temperature at which point the reaction was filtered through a 1” silica plug with 20 mL of Et2O followed by 80 mL of Et2O to elute the remaining product into a 200 mL round bottom flask. After removing the Et2O by rotary evaporation, the crude product was isolated by flash column chromatography.
References:
WO2022/94420,2022,A1 Location in patent:Paragraph 00125; 00135-00136; 00171; 00195; 00201-00203
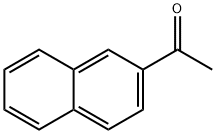
93-08-3
412 suppliers
$6.00/25g

939-27-5
93 suppliers
$16.00/1g
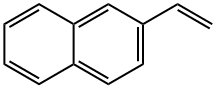
827-54-3
208 suppliers
$45.00/50mg

939-27-5
93 suppliers
$16.00/1g