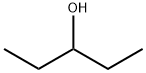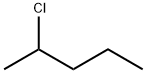
2-CHLOROPENTANE synthesis
- Product Name:2-CHLOROPENTANE
- CAS Number:625-29-6
- Molecular formula:C5H11Cl
- Molecular Weight:106.59

6032-29-7
173 suppliers
$14.00/5mL

625-29-6
37 suppliers
$120.00/25ml
Yield: 83.8%
Reaction Conditions:
Stage #1:(+/-)-2-pentanol with pyridine in N,N-dimethyl-formamide at 0 - 5; for 0.3 h;
Stage #2: with methanesulfonyl chloride in N,N-dimethyl-formamide at 10 - 65; for 11.5 h;Reagent/catalyst;Solvent;
Steps:
1 Example 1
Preparation of 2-chloropentane (5: X=Cl)
2-Pentanol (4) (296.71 g, 3.366 mol), pyridine (479.25 g, 6.06 mol), and N,N-dimethylformamide (DMF) (1009.80 g) were added to a reactor at room temperature and stirred at from 0 to 5° C. for 18 minutes.
Next, methanesulfonyl chloride (MsCl) (6: X=Cl, R=Me) (539.81 g, 4.71 mol) was added dropwise to the reactor at an internal temperature of 10° C. or below.
After the completion of the dropwise addition, the reaction mixture was heated to an internal temperature of from 60 to 65° C. and stirred for 11.5 hours.
After lowering the internal temperature to 30° C., acetic acid (363.83 g) and water (1683.00 g) were added to the reaction mixture to cause phase separation and the aqueous phase thus obtained was removed.
Then, sodium hydrogen carbonate (84.15 g) and water (841.50 g) were added to the organic phase to wash the same, followed by phase separation and removal of the aqueous phase.
The organic phase thus obtained was concentrated at a reduced pressure and the residue was subjected to distillation at a reduced pressure to obtain 2-chloropentane (5: X=Cl) (300.80 g, 2.82 mol) in a yield of 83.8%. 1-Chloropentane and 1-pentene, i.e., regioisomers of 2-chloropentane (5: X=Cl), was not observed in GC analysis.
The following is the spectrum data of the 2-chloropentane (5: X=Cl) thus prepared.
[Nuclear magnetic resonance spectrum] 1H-NMR (500 MHz, CDCl3): δ=0.92 (3H, t, J=7.3 Hz), 1.36-1.58 (2H, m), 1.50 (3H, d, J=4.6 Hz), 1.62-1.76 (2H, m), 4.04 (1H, td, J=1.5, 6.5 Hz); 13C-NMR (500 MHz, CDCl3): δ=13.50, 19.85, 25.33, 42.41, 58.56 [Mass spectrum] EI-Mass spectrum (70 eV); m/z 105 (M+-1), 91, 70, 55, 43, 27 [Infrared absorption spectrum](NaCl): v=2962, 2933, 2875, 1458, 1380, 1270, 746, 672, 614
References:
Shin-Etsu Chemical Co., Ltd.;Miyake, Yuki;Kinsho, Takeshi;Komatsu, Ryo US2020/199053, 2020, A1 Location in patent:Paragraph 0161-0170

109-66-0
441 suppliers
$10.00/25ml

543-59-9
191 suppliers
$20.00/25ml

625-29-6
37 suppliers
$120.00/25ml
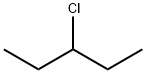
616-20-6
21 suppliers
inquiry

109-68-2
68 suppliers
$40.00/25mL

625-29-6
37 suppliers
$120.00/25ml