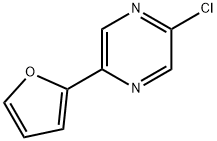
2-CHLORO-5-FURAN-2-YL-PYRAZINE synthesis
- Product Name:2-CHLORO-5-FURAN-2-YL-PYRAZINE
- CAS Number:82619-63-4
- Molecular formula:C8H5ClN2O
- Molecular Weight:180.59
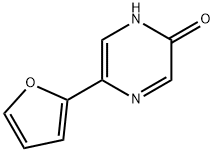
82619-62-3
23 suppliers
$45.00/10mg

82619-63-4
29 suppliers
$45.00/10mg
Yield:82619-63-4 45%
Reaction Conditions:
with trichlorophosphate for 3 h;Heating / reflux;
Steps:
1.C (4-Cyclobutyl-[1,4]diazepan-1-yl)-[5-(4-fluoro-phenoxy)-pyrazin-2-yl]-methanone
Step C: 2-Chloro-5-furan-2-yl-pyrazine. A solution of 5-furan-2-yl-pyrazin-2-ol (7.20 g, 44.4 mmol) in POCl3 (60 mL) was heated at reflux for 3 h. The reaction was allowed to cool, and excess POCl3 was removed by rotary evaporation. The residue was quenched with ice and water. The acidic mixture was basified with aqueous NaOH to pH 10, and the product was extracted with CHCl3. The combined organic extracts were dried (Na2SO4) and concentrated to provide the title chloropyrazine (3.62 g, 45%). 1H NMR (500 MHz, CDCl3): δ 8.73 (d, J=1.4 Hz, 1H), 8.52 (d, J=1.4 Hz, 1H), 7.60 (d, J=1.7 Hz, 1H), 7.14 (d, J=3.4 Hz, 1H), 6.58 (dd, J=3.5, 1.8 Hz, 1H).
References:
US2009/131416,2009,A1 Location in patent:Page/Page column 9
20328-66-9
18 suppliers
inquiry

82619-63-4
29 suppliers
$45.00/10mg