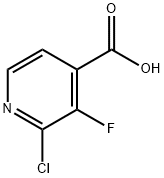
2-Chloro-3-fluoropyridine-4-carboxylic acid synthesis
- Product Name:2-Chloro-3-fluoropyridine-4-carboxylic acid
- CAS Number:628691-93-0
- Molecular formula:C6H3ClFNO2
- Molecular Weight:175.54
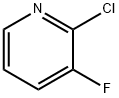
17282-04-1
353 suppliers
$7.00/10g
124-38-9
130 suppliers
$175.00/23402

628691-93-0
176 suppliers
$6.00/1g
Yield:628691-93-0 79%
Reaction Conditions:
Stage #1:2-chloro-3-fluoropyridine with lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 3.33333 h;
Stage #2:carbon dioxide in tetrahydrofuran;n-heptane;ethylbenzene at 0; for 1.33333 h;
Stage #3: with hydrogenchloride in water; pH=2
Steps:
14 EXAMPLE 14 3-(2'-Cyano-2,4'-difluorobiphenyl-5-yl)-8-fluoroimidazo[1,2-a]pyridine-7- carboxylic acid methyl ester
A mixture of lithium diisopropylamide (2M in heptane/ tetrahydrofuran/ethylbenzene-stabilised with magnesium bis- (diisopropylamide), 16. 7 ml) and tetrahydrofuran (30 ml) was cooled to [- 78°C] and [A PRE-COOLED (-78°C)] solution of 2-chloro-3-fluoropyridine (4.0 g, 30.4 mmol) in tetrahydrofuran (30 ml) was added dropwise over 20 min. After 3 h, carbon dioxide was bubbled through the cold reaction mixture for 20 min. The reaction was then warmed [TO-30°C] for 1 h then warmed [TO 0°C.] The mixture was quenched by the addition of water (75 [ML).] The aqueous phase was washed with diethyl ether (100 ml), then the pH of the solution adjusted to 2 by the addition of 2N hydrochloric acid. The resulting white precipitate was aged for 18 h then filtered and left to air- dry, which afforded [2-CHLORO-3-FLUOROISONICOTINIC] acid as a white solid (4.22 g, 79%): 8H (360 MHz, DMSO) 7. 80 [(1H,] dd, J 5 and 5), 8. 39 [(1H,] d, J 5), 14.20 [(1H,] s). [2-CHLORO-3-FLUOROISONICOTINIC] acid (3.30 g, 18.8 mmol) was suspended in thionyl chloride (40 ml) and heated under reflux for 2.5 h. The solvent was evaporated and the residue dried by means of azeotropic removal of water with toluene (100 ml) to afford a pale yellow oil. The oil was dissolved in dichloromethane (20 ml) and cooled to 0°C before methanol (2.42 g, 75.3 mmol) was added dropwise to the solution over 15 min. On complete addition the mixture was allowed to warm to ambient temperature and stirred for [18] h. The solvent was evaporated and the mixture partitioned between water (75 ml) and dichloromethane (100 ml). The aqueous phase was extracted further with dichloromethane (100 ml), the organic layers were combined, washed with brine (75 ml), dried over anhydrous sodium sulphate, filtered and evaporated to give 2-chloro-3- fluoroisonicotinic acid methyl ester (3.32 g, 93%) as a pale yellow solid: [SN] (360 MHz, [CDCL3)] 3.99 (3H, s), 7.70 [(1H,] dd, J 5 and 5), 8. 31 [(1H,] d, J 5). [2-CHLORO-3-FLUOROISONICOTINIC] acid methyl ester (3.32 g, 17.5 mmol) was converted to 8-fluoroimidazo [[L,] 2-a] pyridine-7-carboxylic acid methyl ester (1.4 g, 41%) as described in Example 1. [8-FLUOROIMIDAZO] [1, 2-a] [PYRIDINE-7-CARBOXYLIC] acid methyl ester (0.19 g, 1.0 mmol) was brominated as described in Example [1,] affording 3- [BROMO-8-FLUOROIMIDAZO] [1, 2-a] pyridine-7-carboxylic acid methyl ester (195 mg, 71%): [8H] (360 MHz, CDCl3) 4.00 (3H, s), 7. 32 [(1H,] t, [J 6.] 9), 7.43 (1H, dd, [J 7] and 6), 8. 19 [(1H,] s), 8. 37 [(1H,] d, [J 7).] 3-Bromo-8-fluoroimidazo [[L,] 2-a] pyridine-7-carboxylic acid methyl ester (0.10 g, 0.37 mmol) and 4, [2'-DIFLUORO-5'- (5,] 5-dimethyl- [1, 3,2] [DIOXABORINAN-2-YL) BIPHENYL-2-CARBONITRILE] (0.15 g, 0.44 mmol) were coupled following the procedure in Example 1 to afford 3- (2'-cyano-2, 4'- difluorobiphenyl-5-yl) -8-fluoroimidazo [1, 2-a] pyridine-7-carboxylic acid methyl ester (79 mg, 53%) as a white solid: [6H] (400 MHz, CDCl3) 4.00 (3H, s), 7.35 [(1H,] dd, J 7 and 7), 7.34-7. 46 (2H, [M), 7. 53-7.] 66 (4H, m), 7.85 (1H, [S),] 8. 24 [(1H,] [D,] J [7)] ; m/z [(ES+)] 408 [(100%,] [MHZ+).]
References:
MERCK SHARP & DOHME LIMITED WO2003/99816, 2003, A1 Location in patent:Page 61