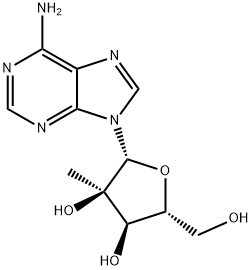
2'-C-Methyladenosine synthesis
- Product Name:2'-C-Methyladenosine
- CAS Number:15397-12-3
- Molecular formula:C11H15N5O4
- Molecular Weight:281.27
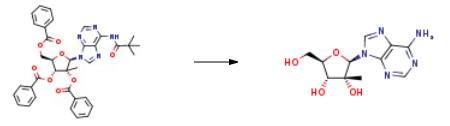
Synthesis of yfl-2'-methyl-adenosine (CHCl); N6-tert-butanoyl-/?-2'-methyl-2',3',5'-tribenzoyl-adenosine (400 mg, 0.590 mmol) was added to a solution of MeOH saturated with ammonia, and stirred at room temperature. After 12 hours the solvent was removed and the obtained solid was purified by column chromatography in gradient starting with a mixture of CHCl3/MeOH 9:1 then 8:2. The pure product was obtained as a white solid (120 mg, 0.427 mmol, 72%). δH (J6-DMSO): 8.47 (IH, s, H8-adenosine), 8.15 (IH, s, H2-adenosine), 7.30 (IH, s, NH26-adenosine), 5.95 (IH, s, Hl '-adenosine), 5.25-5.21 (3H, m, OH5' -adenosine, OH3'- adenosine, OH2' -adenosine), 4.12-4.05 (IH, d, H3 '-adenosine, J= 8.6 Hz), 3.91 (IH, m, H4'-adenosine), 3.84 (IH, m, H5' -adenosine), 3.70 (IH, m, H5' -adenosine), 0.77 (3H3 s, CH32'-adenosine); δc (4-DMSO): 156.02 (1C, C6-adenosine), 152.53 (1C, C2-adenosine), 149.01 (1C, C4-adenosine), 138.68 (1C, C8-adenosine), 118.67 (1C, C5-adenosine), 90.78 (1C, Cl '-adenosine), 82.52 (1C, C4' -adenosine), 78.46 (1C, C2'-adenosine), 71.63 (1C, C3' -adenosine), 59.47 (1C, C5 '-adenosine), 19.83 (1C, CH3-2'-adenosine). Anal. CaIc. for C11H15N5O4: C 46.97%, H 5.38%, N 24.90%. Found: C 46.67%, H 5.22%, N 24.20%.
205171-04-6
21 suppliers
$1066.00/1g

15397-12-3
75 suppliers
$55.00/50mg
Yield:-
Reaction Conditions:
with ammonia in 1,4-dioxane at 110; for 16 h;Sealed tube;
Steps:
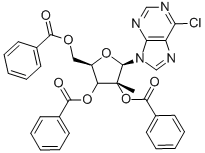
10
To a solution of compound 13-5 (40 g, 62 mmol) in dioxane (50 mL) was added ammonia (30%, 150 mL). The solution was stirred for 16 h at 110 oC in sealed tube. The solution was cooled to R.T., the mixture was concentrated under reduced pressure, washed with EA (2x400 mL). Compound 13-6 was obtained ((2R,3R,4R,5R)-2-(6-amino- 9H-purin-9-yl)-5-(hydroxymethyl)-3-methyltetrahydrofuran-3,4-diol, crude, 16 g) as a white solid. ESI-MS: m/z 282 [M+H]+.
References:
WO2018/31818,2018,A2 Location in patent:Paragraph 0381