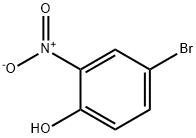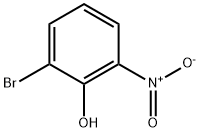
2-Bromo-6-nitrophenol synthesis
- Product Name:2-Bromo-6-nitrophenol
- CAS Number:13073-25-1
- Molecular formula:C6H4BrNO3
- Molecular Weight:218
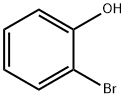
95-56-7
302 suppliers
$9.00/5 g

13073-25-1
332 suppliers
$6.00/1g
Yield:13073-25-1 42.8%
Reaction Conditions:
with sodium nitrate;sulfuric acid in water at 20 - 25;
Steps:
1.1
Step 1 2-Bromo-6-nitro-phenol; A solution of 60 mL of concentrated sulfuric acid diluted with 186 mL of water was cooled to room temperature. Sodium nitrate (79.2 g, 0.932 mol) was added to the solution. 2-Bromo-phenol 1a (60 mL, 0.516 mol) was added dropwise at such a rate that the reaction temperature was kept below 25 °C. The reaction mixture was reacted at room temperature for 2 hours and monitored by thin layer chromatography (TLC) until the disappearance of the starting materials. The precipitate was dissolved in 320 mL of ethyl acetate. The mixture was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound 2-bromo-6-nitro-phenol 1b (48.2 g, yield 42.8%) as a yellow solid. MS m/z (ESI): 218 [M+1] 1H NMR (400 MHz, CDCl3): δ 6.88~7.02 (m, 1H), 7.89~7.91 (d, J = 8 Hz, 1H), 8.12~8.15 (m, 1H), 11.18 (s, 1H)
References:
Jiangsu Hengrui Medicine Co., Ltd.;Shanghai Hengrui Pharmaceutical Co. Ltd. EP2236500, 2010, A1 Location in patent:Page/Page column 15
88-75-5
12 suppliers
$15.00/5mg

13073-25-1
332 suppliers
$6.00/1g
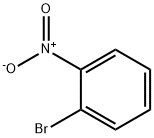
577-19-5
8 suppliers
$14.00/5g

13073-25-1
332 suppliers
$6.00/1g
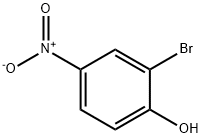
5847-59-6
164 suppliers
$9.00/1g
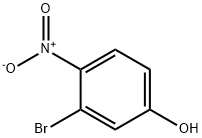
5470-65-5
99 suppliers
$73.00/250mg
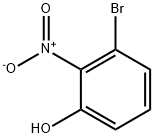
76361-99-4
101 suppliers
inquiry