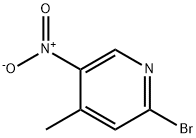
2-Bromo-5-nitro-4-picoline synthesis
- Product Name:2-Bromo-5-nitro-4-picoline
- CAS Number:23056-47-5
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
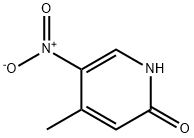
21901-41-7
278 suppliers
$6.00/1g

23056-47-5
261 suppliers
$10.00/1g
Yield:23056-47-5 90%
Reaction Conditions:
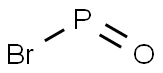
with phosphorus(V) oxybromide in dichloromethane for 24 h;Heating / reflux;
Steps:
1.1.A
A solution of 4-methyl-5-nitropyridin-2-ol (82 g, 0.53 mol) and POBr3 (229 g, 0.8 mol, 1.5 eq. ) in dichloromethane (2 L) was heated under a nitrogen atmosphere in a 3 L 3-neck flask with overhead stirrer for 24 h. The mixture was filtered cold. The solid consisted of a 1: 1 mixture of desired product 2 and unreacted starting material 4-methyl-5- nitropyridin-2-ol, the filtrate contained the desired product and unreacted POBr3. The solid (46 g) and fresh POBr3 were dissolved in dichloromethane and refluxed for 10 h. The liquid was decanted and combined with the filtrate of the first reaction. The combined solutions were concentrated to a volume of 1.5 L. The mixture was cooled in an ice bath and stirred while ice (500 g) was added to quench the excess POBr3. The organic layer was separated, and the aqueous layer was extracted with dichloromethane. The combined organic extracts were dried (magnesium sulfate) and concentrated. The product was recrystallized from hexanes. Yield: 104 g (90%). 1H NMR (300 MHz, CDC13) No. ppm 2.63 (s, 3 H) 7.52 (s, 1 H) 8.95 (s, 1 H). LCMS: (APCI, M-H') = 215 and 217 u/e (bromine isotope pattern) ; Retention Time: 3.01 min; Purity by TIC: 95%; Purity by UV: 95%.
References:
PFIZER INC. WO2005/103003, 2005, A2 Location in patent:Page/Page column 61
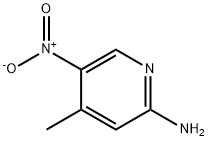
21901-40-6
293 suppliers
$6.00/1g

23056-47-5
261 suppliers
$10.00/1g
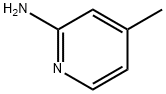
695-34-1
530 suppliers
$5.00/10g

23056-47-5
261 suppliers
$10.00/1g