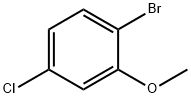
2-BROMO-5-CHLOROANISOLE synthesis
- Product Name:2-BROMO-5-CHLOROANISOLE
- CAS Number:174913-09-8
- Molecular formula:C7H6BrClO
- Molecular Weight:221.48
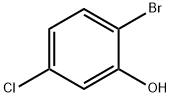
13659-23-9
179 suppliers
$19.00/1g

74-88-4
346 suppliers
$15.00/10g

174913-09-8
200 suppliers
$6.00/1g
Yield:174913-09-8 97%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide; for 2 h;
Steps:
27
Preparation Example 27 Synthesis of 1-bromo-4-chloro-3-(4-ethoxybenzyl)-6-methoxybenzene; A suspension of 2-bromo-5-chlorophenol (2.85 g, 13.7 mmol; synthesized in reference to International Patent Publication WO0109122), potassium carbonate (1.89 g, 13.7 mmol), n-BU4NI (50 mg, 0.137 mmol), methyl iodide (1.28 mL, 20.6 mmol) and N,N-dimethylformamide (8.0 mL) was stirred for two hours. An iced water was added and the obtained mixture was extracted with ethyl acetate twice. The combined organic phase was washed with brine and dried with anhydrous magnesium sulfate. After the desiccant was filtered off, the residue obtained by evaporating the solvent under reduced pressure was purified by silica gel column chromatography (hexane:ethyl acetate=95:5) to obtain colorless oily 2-bromo-5-chloroanisole (2.94 g, 97%). Then, oxalyl chloride (1.23 mL, 15.1 mmol) and N,N-dimethylformamide (2 drops) were added to 4-ethoxybenzoic acid (2.28 g, 13.7 mmol) in chloroform (8 mL) and stirred for five hours. The yellow oil obtained by evaporating the solvent under reduced pressure was dissolved in chloroform (5 mL). To this solution, a chloroform solution (10 mL) of 2-bromo-5-chloroanisole (2.94 g, 13.3 mmol) was added and then aluminum chloride (2.07 g, 15.5 mmol) was added portion wise at -10°C over five minutes. After stirred at 5°C for one hour, the reaction mixture was to room temperature and stirred for 13 hours. The reaction mixture was poured into an iced water and extracted with chloroform three times. After washed with 1 M hydrochloric acid, water, brine, the combined organic layer was dried with anhydrous magnesium sulfate. After the desiccant was filtered off, the residue obtained by evaporating the solvent under reduced pressure was purified by NH type silica gel column chromatography (hexane:ethyl acetate=9:1) to obtain (5-bromo-2-chloro-6-methoxyphenyl)(4-ethoxyphenyl)methanone (1.53 g, 31%) as a colorless crystal. Then, Et3SiH (1.62 mL, 10.1 mmol) and BF3·Et2O (0.772 mL, 6.09 mmol) were added sequentially to a chloroform - acetonitrile (1:1; 16 mL) solution of (5-bromo-2-chloro-6-methoxyphenyl)(4-ethoxyphenyl)methanone (1.50 g, 4.06 mmol) at -5°C. The reaction mixture was warmed to room temperature and stirred for 16 hours. After the reaction mixture was added with a saturated sodium carbonate aqueous solution and extracted with chloroform, the organic layer was washed with brine and dried with anhydrous magnesium sulfate. After the desiccant was filtered off, the residue obtained by evaporating the solvent under reduced pressure was purified by silica gel column chromatography (hexane:ethyl acetate=20:1) to obtain a colorless oily title compound (1.48 g, 99%). 1H NMR (200 MHz, CHLOROFORM-d) δ ppm 1.40 (t, J=7.0 Hz, 3 H) 3.87 (s, 3 H) 3.93 (s, 2 H) 4.01 (q, J=7.0 Hz, 2 H) 6.77 - 6.87 (m, 2 H) 6.90 (s, 1 H) 7.03 - 7.12 (m, 2 H) 7.29 (s, 1 H). EI 354(M+), 356(M+2), 358(M+4).
References:
EP1845095,2007,A1 Location in patent:Page/Page column 41-42
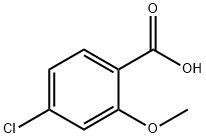
57479-70-6
168 suppliers
$10.00/1g

174913-09-8
200 suppliers
$6.00/1g

13659-23-9
179 suppliers
$19.00/1g

77-78-1
302 suppliers
$22.00/25g

174913-09-8
200 suppliers
$6.00/1g