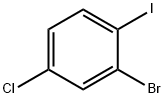
2-Bromo-4-chloro-1-iodobenzene synthesis
- Product Name:2-Bromo-4-chloro-1-iodobenzene
- CAS Number:31928-44-6
- Molecular formula:C6H3BrClI
- Molecular Weight:317.35
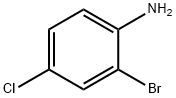
873-38-1
286 suppliers
$6.00/5g

31928-44-6
178 suppliers
$6.00/1g
Yield:31928-44-6 81%
Reaction Conditions:
Stage #1: 4-chloro-2-bromoanilinewith hydrogenchloride;sodium nitrite in water at -15 - 0; for 0.833333 h;
Stage #2: with potassium iodide in water at 20; for 6.17 h;
Steps:
4
[Production Example 4]; The following Indenopyrene Compound D was produced through the following synthesis route. [Show Image] Synthesis of Intermediate D1; 2-Bromo-4-chloroaniline (10 g, 48 mmoles) was suspended in hydrochloric acid water (50 mL of concentrated hydrochloric acid and 35 mL of water), and the suspension was cooled on a dry ice/methanol bath at -15°C. A sodium nitrite aqueous solution (3.6 g, 52 mmoles, 1. 1 eq. /20 mL) was gradually added dropwise thereto over 20 minutes, and the mixture was stirred at from -15°C to 0°C for 30 minutes, thereby preparing a diazonium salt. The reaction solution was gradually added dropwise to a potassium iodide aqueous solution (73 g, 0.44 moles, 9 eq. /220 mL) at room temperature over 10 minutes. The reaction mixture was stirred at room temperature for 6 hours and then allowed to stand overnight. Dichloromethane (200 mL) was added to the reaction mixture, and subsequently, sodium hydrogensulfite (2 g) was added, thereby deactivating generated iodine. An organic layer was aliquoted, washed with a 10% sodium hydrogensulfite aqueous solution (100 mL) and saturated salt water (30 mL) and dried over anhydrous magnesium sulfate, and the solvent was then distilled off to obtain a red liquid. This was purified by means of column chromatography (silica gel/hexane) to obtain a white needle crystal (12.4 g, 81%). 1H-NMR (400 MHz, CDCl3, TMS): δ6.98 (1H, dd, J = 8 Hz, 2 Hz), 7.61 (1H, d, J = 2 Hz), 7.74 (1H, d, J = 8 Hz)
References:
EP2316816,2011,A1 Location in patent:Page/Page column 21
![Pyridine, 2-[(2-bromo-4-chlorophenyl)bis(1-methylethyl)silyl]-](/CAS/20210305/GIF/1260425-79-3.gif)
1260425-79-3
0 suppliers
inquiry

31928-44-6
178 suppliers
$6.00/1g
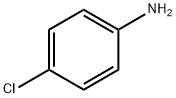
106-47-8
474 suppliers
$5.00/5G

31928-44-6
178 suppliers
$6.00/1g
![Pyridine, 2-[(4-chlorophenyl)bis(1-methylethyl)silyl]-](/CAS/20210305/GIF/1232693-03-6.gif)
1232693-03-6
0 suppliers
inquiry

31928-44-6
178 suppliers
$6.00/1g
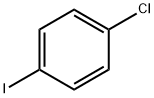
637-87-6
291 suppliers
$38.00/10g

31928-44-6
178 suppliers
$6.00/1g