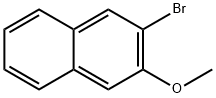
2-Bromo-3-methoxynaphthalene synthesis
- Product Name:2-Bromo-3-methoxynaphthalene
- CAS Number:68251-77-4
- Molecular formula:C11H9BrO
- Molecular Weight:237.09
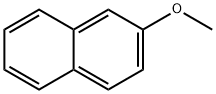
93-04-9
458 suppliers
$13.00/25g

106-93-4
385 suppliers
$15.00/5g

68251-77-4
70 suppliers
$22.00/250mg
Yield:68251-77-4 58.4%
Reaction Conditions:
Stage #1:2-Methoxynaphthalene with n-butyllithium in tetrahydrofuran at -78; for 0.166667 h;
Stage #2:ethylene dibromide in tetrahydrofuran at 20; for 16 h;
Steps:
533.a.n.d.534 Intermediate AZ: 2-bromo-3-methoxynaphthalene
A RBF was charged 2-methoxynaphthalene (2.00 g, 12.64 mmol) and THF (30 mL) to give a clear solution. n-butyllithium (2.5M in hexanes) (5.66 mL, 14.16 mmol) was added dropwise.
The flask was cooled in a dry ice-acetone bath for 10 min and 1,2-dibromoethane (1.634 mL, 18.96 mmol) was added dropwise.
The reaction was warmed to room temperature and stirred for 16 hours.
NaOH (1 M, 10 mL, 10 mmol 0 was added and the reaction was heated to reflux for 1 hour.
The mixture was cooled to room temperature and extracted with DCM (2*), and the combined organic extracts were dried over magnesium sulfate, filtered, and concentrated.
The crude solid was recrystallized from heptane to give 2-bromo-3-methoxynaphthalene (1.75 g, 7.38 mmol, 58.4% yield) as an off-white solid. m/z (ESI) 237.1 (M+H)+.
References:
Amgen Inc.;Weiss, Matthew;Boezio, Alessandro;Boezio, Christiane;Butler, John R.;Chu-Moyer, Margaret Yuhua;Dimauro, Erin F.;Dineen, Thomas;Graceffa, Russell;Guzman-Perez, Angel;Huang, Hongbing;Kreiman, Charles;La, Daniel;Marx, Isaac E.;Milgrim, Benjamin Charles;Nguyen, Hanh Nho;Peterson, Emily;Romero, Karina;Sparling, Brian US9212182, 2015, B2 Location in patent:Page/Page column 221; 222

93-04-9
458 suppliers
$13.00/25g

68251-77-4
70 suppliers
$22.00/250mg
135-19-3
734 suppliers
$9.00/1g

68251-77-4
70 suppliers
$22.00/250mg
13041-60-6
55 suppliers
$60.00/50mg

68251-77-4
70 suppliers
$22.00/250mg
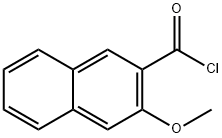
58778-69-1
6 suppliers
inquiry

68251-77-4
70 suppliers
$22.00/250mg