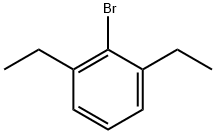
2-BROMO-1,3-DIETHYLBENZENE synthesis
- Product Name:2-BROMO-1,3-DIETHYLBENZENE
- CAS Number:65232-57-7
- Molecular formula:C10H13Br
- Molecular Weight:213.11
579-66-8
287 suppliers
$19.00/25mL

65232-57-7
61 suppliers
$24.00/250mg
Yield: 83%
Reaction Conditions:
Stage #1:2,6-diethylaniline with hydrogen bromide in water at 20; for 0.25 h;
Stage #2: with sodium nitrite in diethyl ether;water at -55 - -18; for 2.25 h;
Steps:
22.a Synthesis of 2-bromo-1 ,3-diethyl-benzene
265 g (1.57 mol) of 48% HBr solution are slowly added to 30.5 g (0.20 mol) of2,6-diethylaniline at room temperature during 15 minutes by carefully controlling thetemperature. The beige suspension is cooled down to -55°C and 23.8 g (0.34 mol) of sodium nitrite are carefully added in small portions at a maximum temperature of -48°C during 40 minutes. The brown suspension is further stirred at -53°C during 50 minutes. 250 ml of pre-cooled diethyl ether are slowly added during 15 minutes and the temperature slowly increased to -18°C during 30 minutes until no more gas is released (careful controlof gas evolution). The brown suspension is cooled down to -54°C and slowly treated with25 g of water and 119 g (0.41 mol) of sodium carbonate decahydrate. The temperature is let rising to room temperature during four hours carefully controlling the amount of gas released. The suspension is further stirred at room temperature during 19 hours. The organic phase is separated, extracted with water (2x 100 ml), dried over sodium sulfatedand concentrated under vacuum. The crude product is further purified by chromatography (silica gel, heptane) giving the title product as a yellow oil (yield: 36.3 g (83%)).1HNMR (400 MHz, CD2CI2): = 1.35 (t, 6 H), 2.91 (q, 4 H), 7.17 (d, 2 H), 7.27 (dd, I H).
References:
BASF SE;MURER, Peter;BATTAGLIARIN, Glauco;DORMANN, Korinna;METZ, Stefan;WAGENBLAST, Gerhard;BENEDITO, Flavio Luiz;WATANABE, Soichi;LENNARTZ, Christian;GESSNER, Thomas WO2015/14835, 2015, A1 Location in patent:Page/Page column 178