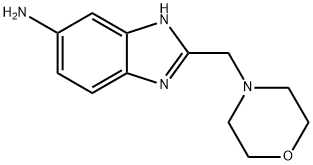
2-BENZYL-1H-BENZOIMIDAZOL-5-YLAMINE synthesis
- Product Name:2-BENZYL-1H-BENZOIMIDAZOL-5-YLAMINE
- CAS Number:313518-70-6
- Molecular formula:C12H16N4O
- Molecular Weight:232.28
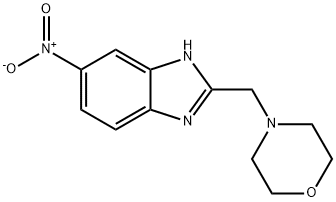
94720-52-2
7 suppliers
inquiry

313518-70-6
21 suppliers
$701.00/5g
Yield:-
Reaction Conditions:
with hydrogen;palladium on activated carbon in ethanol;
Steps:
573 N-(2-Chloro-6-methylphenyl)-2-[[2-(4-morpholinylmethyl)-1H-benzimidazol-5-yl]amino]-5-thiazolecarboxamide
[0637] A mixture of 3,4-diamino-nitrobenzene (15.3 g, 0.10 mole) and chloroacetic acid (14.18 gm, 1.5 eq) in 5.0 N aqueous HCl (80 mL) was heated at reflux for 1 hr. After cooling to RT, the reaction was filtered through celite and the filtrate was stored at 0° C. for 2 days. The crystals that formed, were collected and recrystallized from a mixture of EtOH and water to give 7.2 gm of the hydrogen chloride salt of 2-chloromethyl-5-nitro-benzimidazole. A portion of this salt (528 mg, 2.13 mmole) and morpholine (1.31 mL, 7 eq) in toluene (15 mL) were heated at reflux for 4 hr. After cooling to RT, the reaction was filtered and the filter cake was washed with toluene. The solvent was removed from the filtrate to leave the crude 2-[N-morpholinylmethyl]-5-nitro-benzimidazole as an oil. A portion of this material (657 mg) and 10% palladium on charcoal (650 mg) in EtOH (10 mL) was stirred overnight under a hydrogen atmosphere (balloon). Removal of the catalyst by filtration and the solvent left the crude 5-amino-2-[N-morpholinylmethyl]-benzimidazole as an oil. A portion of this material was coupled with 556 as described for 570 to afford 573 (HPLC retention time (YMC ODS S7 3×50 mm): 0.92 min).
References:
US2004/54186,2004,A1 Location in patent:Page 146-147