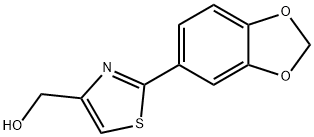
(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL synthesis
- Product Name:(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL
- CAS Number:248249-56-1
- Molecular formula:C11H9NO3S
- Molecular Weight:235.26
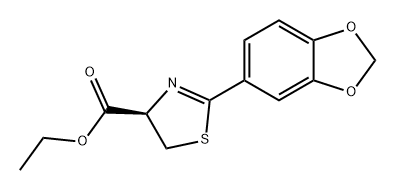
![2-BENZO[1,3]DIOXOL-5-YL-THIAZOLE-4-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/248249-53-8.gif)
248249-53-8
25 suppliers
$87.50/250mg
![(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL](/CAS/GIF/248249-56-1.gif)
248249-56-1
13 suppliers
inquiry
Yield:248249-56-1 64%
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran; for 2 h;Heating / reflux;
Steps:
20 Example 20, Synthesis of 2-(benzo[3,4-d]1,3-dioxolan-5-yl)-4-hydroxymethyl-1,3-thiazole(Compound 25)
Ethyl 4-(benzo[3,4-d]1,3-dioxolan-5-yl)-3,5-thiazole carboxylate prepared in Example 17(1.93g, 6.96mmol) was dissolved in tetrahydrofuran(THF, 30mlitre), borane-methylsulfide complex(10M in THF, 2.1mlitre, 20.88mmol) was added thereto, and the mixture was stirred under reflux for 2 hours. The reaction vessel was cooled down to 0°C, and the reaction was stopped by slowly adding 1N-HCl. The pH of the reaction solution was adjusted to 6-7 using saturated sodium bicarbonate solution, and then the organic solvent was removed under reduced pressure. The residue was extracted with ethyl acetate, and then the extract was washed with saturated saline solution, dried over anhydrous magnesium sulfate, and concentrated. The concentrate was recrystallized from ethyl acetate and n-hexane(EA/HA=1/1, v/v) to give the title compound(1.04g, Yield 64%) as a white solid. 1H NMR(300 MHz, CDCl3) : δ 2.46(bs, 1H), 4.79(s, 2H), 6.03(s, 2H), 6.88(dd, 1H, J = 3.0, 5.6 Hz), 7.13(s, 1H), 7.48(dd, 1H, J = 1.7, 5.5 Hz)
References:
EP1073428,2004,B1 Location in patent:Page 29
248249-49-2
0 suppliers
inquiry
![(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL](/CAS/GIF/248249-56-1.gif)
248249-56-1
13 suppliers
inquiry
55308-42-4
0 suppliers
inquiry
![(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL](/CAS/GIF/248249-56-1.gif)
248249-56-1
13 suppliers
inquiry
4421-09-4
152 suppliers
$8.00/1g
![(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL](/CAS/GIF/248249-56-1.gif)
248249-56-1
13 suppliers
inquiry
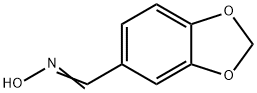
2089-36-3
46 suppliers
$47.70/5g
![(2-BENZO[1,3]DIOXOL-5-YL-THIAZOL-4-YL)-METHANOL](/CAS/GIF/248249-56-1.gif)
248249-56-1
13 suppliers
inquiry