
2-Aminonaphthalene-1-sulfonic acid synthesis
- Product Name:2-Aminonaphthalene-1-sulfonic acid
- CAS Number:81-16-3
- Molecular formula:C10H9NO3S
- Molecular Weight:223.25

110-91-8
687 suppliers
$9.00/1g
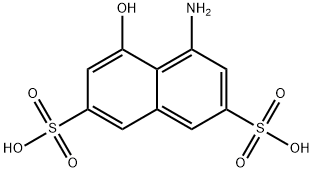
90-20-0
233 suppliers
inquiry
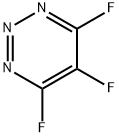
112291-51-7
0 suppliers
inquiry

81-16-3
327 suppliers
$21.00/5g
Yield:-
Reaction Conditions:
with sodium carbonate in water
Steps:
1 EXAMPLE 1
EXAMPLE 1 31.9 g of 1-hydroxy-8-aminonaphthalene-3,6-disulphonic acid are dissolved in 400 ml of water under neutral conditions. At 0° to 5° C., 8.8 ml of trifluorotriazine are added, and the pH is maintained at 4.0 to 4.5 by adding 20% strength sodium carbonate solution. After 5 minutes, 9 g of morpholine are added, and the pH is maintained at 7 using 20% strength sodium carbonate solution. After 15 minutes at 10° C., the reaction is complete. The solution thus obtained of the compound of the formula STR9 is further reacted as follows: A diazonium salt suspension obtained by diazotisation of 22.3 g of 2-aminonaphthalene-1-sulphonic acid prepared in the usual manner is added to the reaction product obtained at 0° to 5° C.
References:
Bayer Aktiengesellschaft US5239063, 1993, A
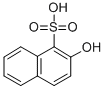
567-47-5
3 suppliers
inquiry

81-16-3
327 suppliers
$21.00/5g
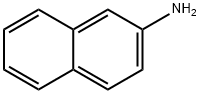
91-59-8
204 suppliers
$54.00/100mg

81-16-3
327 suppliers
$21.00/5g