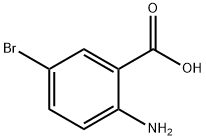
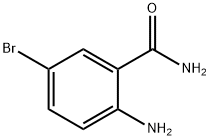
2-Amino-5-bromobenzoic acid synthesis
- Product Name:2-Amino-5-bromobenzoic acid
- CAS Number:5794-88-7
- Molecular formula:C7H6BrNO2
- Molecular Weight:216.03
Synthesis of 2-amino-5-bromobenzoic acid (4c, CAS: 5794-88-7) and 2-amino3,5-dibromobenzoic acid (4d, CAS: 609-85-8): A solution of bromine (7.2 g, 2 mL, 40 mmol) in 47 mL glacial acetic acid was added dropwise to sodium 2-aminobenzoate (6.4 g, 40 mmol) in 32 mL of glacial acetic acid at 15 °С and the mixture was stirred for 1 h at the same temperature. The product was filtered off, washed with benzene and dried in the dark. The bromobenzoic acids containing mixture (0.5 g) was added to 10 mL of boiling water followed by the addition of 1.3 mL of concentrated hydrochloric acid and hot filtration under vacuum. The insoluble material contained 2-amino-3,5-dibromobenzoic acid, whereas 2- amino-5-bromobenzoic acid precipitated upon cooling of the filtrate.
2-Amino-5-bromobenzoic acid (4c) IR (suspension in nujol, cm?1 ): 3497, 3383, 1675, 1616, 1587, 1548, 1423, 1377, 1316, 1292, 1239, 1160, 1127, 812, 748, 691.
87-48-9
289 suppliers
$10.00/5g

5794-88-7
468 suppliers
$6.00/10g
Yield:5794-88-7 96%
Reaction Conditions:
with dihydrogen peroxide;sodium hydroxide in water at 80; for 1 h;
Steps:
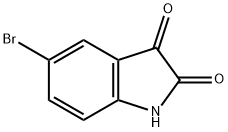
37.A Part A. 2-Amino-5-bromobenzoic acid
To a stirred solution of 5-bromoindoline-2,3-dione (20.0 g, 8.8 mmol) in aq. 3N NaOH (88 mL) stirred at 80 °C, was added dropwise hydrogen peroxide (22.2 mL, 2.7 mmol, 35% in water) and the reaction mixture was stirred at 80 °C for 1 h. The reaction mixture was allowed to cool to room temperature. The reaction mixture was cooled to 0 °C and was quenched by addition of conc. HC1 (100 mL) and the pH of the reaction was adjusted to pH 5. The volatiles were evaporated to dryness under reduced pressure to afford crude product as a brown solid. The solid was suspended in MeOH (150 mL) and stirred for 15 minutes, filtered and the filtrate was evaporatedto dryness under reduced pressure to give 2-amino-5-bromobenzoic acid (18 g, 84.4 mmol, 96% yield) as brown solid. LCMS (ESI) m/e 216.0 [(M+H), calcd for C7H7BrNO2, 216.0]; LC/MS retention time (method A): ti = 0.86 mm.
References:
BRISTOL-MYERS SQUIBB COMPANY;HARTZ, Richard A.;AHUJA, Vijay T.;MACOR, John E.;BRONSON, Joanne J.;DASGUPTA, Bireshwar;DZIERBA, Carolyn Diane;NARA, Susheel Jethanand;KARATHOLUVHU, Maheswaran Sivasamban WO2016/53794, 2016, A1 Location in patent:Page/Page column 131; 132

52727-57-8
232 suppliers
$5.00/1g

5794-88-7
468 suppliers
$6.00/10g
118-92-3
4 suppliers
$21.60/25g

5794-88-7
468 suppliers
$6.00/10g
6950-43-2
114 suppliers
$6.00/250mg

5794-88-7
468 suppliers
$6.00/10g
16313-66-9
95 suppliers
$5.00/100mg

5794-88-7
468 suppliers
$6.00/10g