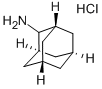
2-Adamantanamine hydrochloride synthesis
- Product Name:2-Adamantanamine hydrochloride
- CAS Number:10523-68-9
- Molecular formula:C10H18ClN
- Molecular Weight:187.71
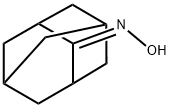
4500-12-3
101 suppliers
$72.00/2 g
700-57-2
306 suppliers
$10.00/1g

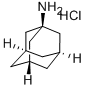
10523-68-9
218 suppliers
$7.00/250mg
Yield:10523-68-9 71%
Reaction Conditions:
Stage #1:2-adamantanone oxime with NiAl2;potassium hydroxide in tetrahydrofuran;waterReflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;tert-butyl methyl ether
Steps:
(1RS,2RS)-Norcamphane-2-amine hydrochloride (19).
General procedure: A 1 M solution of KOH in water, 250 mL, was added to a solution of 5.7 g (~ 46 mmol) of oxime 9 in 50 mL of THF. Nickel-aluminum alloy, 10 g, was added to the resulting mixture in 2.5-g portions in 20-min intervals under vigorous stirring. As this took place, the reaction mixture slightly boiled due to heat release. After all alloy had been added, the reaction mixture was stirred for 30 min and then an additional 2 h under reflux with vigorous stirring. The heating was then turned off, the reaction mixture was cooled to room temperature with stirring, diluted with 50 mL of THF, and filtered. The catalyst that stayed on the filter (prone to self-ignition) was washed with THF (3 × 50 mL) and immediately poured into water. The filtrate was extracted with t-BuOMe, and the extracts were combined and dried with KOH under reflux. The solution was cooled down, decanted from the dryer and evaporated first under normal and then under reduced pressure in a water bath with protection from air CO2. The mixture of amines and norborneols in the still bottom was dissolved in a mixture of dry t-BuOMe and THF and treated by a stream of dry HCl with stirring until weakly acidic. A voluminous white precipitate of amine 19 hydrochloride formed and was filtered off, washed on the filter with dry t-BuOMe, and dried on the filter to constant weight. Yield 2.8 g (42%). The content of the main product 14 isolated by treatment with alkali of the precipitated salt was 100% (by GLC).
References:
Brunilin, R. V.;Mkrtchyan, A. S.;Nawrozkij, M. B.;Novakov, I. A.;Vernigora, A. A.;Voloboev, S. N.;Vostrikova, O. V. [Russian Journal of Organic Chemistry,2019,vol. 55,# 11,p. 1742 - 1748][Zh. Org. Khim.,2019,vol. 55,# 11,p. 1781 - 1788,8]
281-23-2
497 suppliers
$5.00/25g
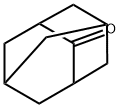
665-66-7
590 suppliers
$16.00/5g

10523-68-9
218 suppliers
$7.00/250mg
700-58-3
455 suppliers
$5.00/5g

700-57-2
306 suppliers
$10.00/1g

10523-68-9
218 suppliers
$7.00/250mg