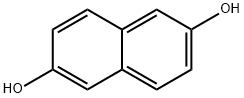
2,6-Naphthalenediol synthesis
- Product Name:2,6-Naphthalenediol
- CAS Number:581-43-1
- Molecular formula:C10H8O2
- Molecular Weight:160.17
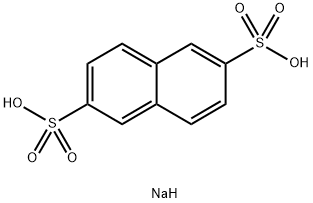
1655-45-4
269 suppliers
$12.00/25g

581-43-1
276 suppliers
$6.00/250mg
Yield:581-43-1 96.9%
Reaction Conditions:
with potassium hydroxide at 200; for 4 h;Reagent/catalyst;
Steps:
1.S2-3.S2 [Example 1]
S2. Add 200g of potassium hydroxide into the grinding vacuum reactor, add 1g of Na-ZnO/Al2O3 solid super alkali prepared in step S1, after fully grinding, then add 100g of 2,6-naphthalene disulfonic acid sodium salt in batches, and heat up Grind the reaction sufficiently to 200°C. The reaction time is 4 hours. After the reaction is complete, the discharging is diluted with ice water. After cooling, it is neutralized with 10% dilute hydrochloric acid until the solution is acidic. The precipitate is precipitated, filtered and dried to obtain 2 ,6-Dihydroxynaphthalene products; Dissolve the 2,6-dihydroxynaphthalene product in 150mL of boiling water, add activated carbon and stir to decolorize, filter while it is hot, cool the filtrate to crystallize, filter, take the filter cake and add it to 50ml methanol, heat to 4050, and wait for all to dissolve Then, a certain amount of water was slowly added, the temperature was lowered to 0° C., the cooling and crystallization were performed, and 46.7 g of high-purity 2,6-dihydroxynaphthalene was obtained by vacuum drying, with a yield of 96.9% and a purity of 99.4%.
References:
CN112299956, 2021, a Location in patent:Paragraph 0025; 0028-0030; 0032-0034; 0036-0038; 0040
121807-17-8
9 suppliers
inquiry

581-43-1
276 suppliers
$6.00/250mg

24157-81-1
113 suppliers
$33.00/25g

581-43-1
276 suppliers
$6.00/250mg
91909-30-7
0 suppliers
inquiry
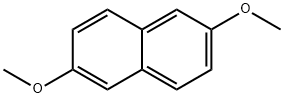
5486-55-5
180 suppliers
$45.00/50mg

581-43-1
276 suppliers
$6.00/250mg
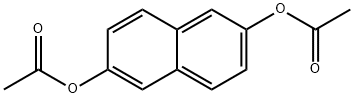
22426-47-7
97 suppliers
$59.00/2 g

581-43-1
276 suppliers
$6.00/250mg
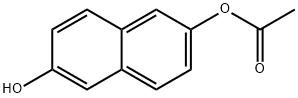
125363-65-7
1 suppliers
inquiry