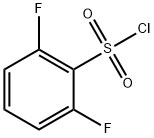
2,6-Difluorobenzenesulfonyl chloride synthesis
- Product Name:2,6-Difluorobenzenesulfonyl chloride
- CAS Number:60230-36-6
- Molecular formula:C6H3ClF2O2S
- Molecular Weight:212.6
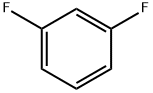
372-18-9
422 suppliers
$9.00/1g

60230-36-6
243 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
Stage #1: 1,3-Difluorobenzenewith n-butyllithium in diethyl ether;hexane at -78; for 3 h;
Stage #2: with sulfur dioxide in diethyl ether;hexane at -60; for 0.333333 h;
Stage #3: with N-chloro-succinimide in diethyl ether;hexane at -60 - 20; for 1 h;
Steps:
1
To a cooled solution of 1,3-difluorobenzene (9.4 g, 82 mmol) in diethyl ether (120 mL) was added n-BuLi in hexane (32.3 mL, 81 mmol) dropwise at -78° C. The reaction mixture was stirred at -78° C. for 3 h. Sulfur dioxide (106 g, 1648 mmol) was flushed into the solution and stirred at -60° C. for 20 mins, which produced a white solid. NCS (12.10 g, 91 mmol) was added and the reaction mixture was warmed to rt and for 1 h. The reaction mixture with white solid changed to pale brown solution. The reaction mixture was filtered and concentrated to give crude No.34 2,6-difluorobenzenesulfonyl chloride, which was added dropwise into No.35 methyl 3-amino-2-fluorobenzoate (5.19 g, 30.7 mmol) in No.36 pyridine (50 mL) at 15° C. After the addition, the reaction mixture was stirred at room temperature overnight. No.38 Ethyl acetate (100 mL) was added to the reaction mixture and then followed by 40 mL of cold No.10 water. The mixture was heated to 55° C. with stirring until all red solids dissolved. The upper layer was organic layer which was isolated and washed again with water. The organic layer was dried and evaporated under reduced pressure. Petro ether was added to the crude product and heated to reflux and then cooled to 5° C. The solids were filtered and rinsed with petro ether to afford No.39 methyl 3-(2,6-difluorophenylsulfonamido)-2-fluorobenzoate (6.91 g, 10.35 mmol, 12.56% yield) as orange solid. LCMS: [M+NH4]+=363.0.
References:
US2013/53562,2013,A1 Location in patent:Paragraph 0073