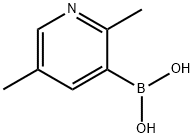
2,5-DIMETHYLPYRIDINE-3-BORONIC ACID synthesis
- Product Name:2,5-DIMETHYLPYRIDINE-3-BORONIC ACID
- CAS Number:1029654-18-9
- Molecular formula:C7H10BNO2
- Molecular Weight:150.97
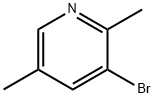
17117-19-0
69 suppliers
$224.00/1g

1029654-18-9
22 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2,5-dimethyl-3-bromopyridinewith n-butyllithium in hexanes;diethyl ether at -78; for 1 h;Inert atmosphere;
Stage #2: with Triisopropyl borate in hexanes;diethyl ether at 20;
Stage #3: with ammonium chloride in hexanes;diethyl ether;
Steps:
99.4
n-BuLi (1.6 M in hexanes, 6.7 ml_, 10.8 mmol, 2.0 equiv) was added dropwise to a cold (- 78°C) solution of 3-bromo-2,5-dimethyl-pyridine (Bulletin de Ia Societe Chimique de France, 1972, (6), 2466-81 ) (1 g, 5.38 mmol) in Et2O (20 ml_), under an argon atmosphere. The reaction mixture was stirred for 1 h at -78°C. Triisopropyl borate (3.7 ml_, 16.1 mmol, 3.0 equiv) was then added. The reaction mixture was allowed to warm to rt, quenched by addition of a saturated solution of NH4CI (1 ml_), and concentrated. The residue was diluted with EtOAc/H2O and the pH adjusted to 7. The aqueous layer was separated and extracted with EtOAc. The organic phase was dried (Na2SO4), filtered and concentrated to afford 170 mg of the title compound as a beige solid (batch 1 ). The aqueous layer was concentrated, the residue combined with batch 1 and dissolved in EtOH (5 ml_). This solution was added to a mixture of 5,8-dibromo-quinoxaline (800 mg, 2.8 mmol) (Step 1.5), PdCI2(dppf) (1 13 mg, 0.1 mmol, 0.05 equiv), Na2COs (2 M solution in H2O, 5.6 ml_, 1 1.1 mmol, 4 equiv) in toluene (30 ml.) at 1050C, under an argon atmosphere. The reaction mixture was stirred at 105 0C for 4 h, allowed to cool to rt, diluted with EtOAc and H2O, and extracted with EtOAc. The organic phase was washed with H2O and brine, dried (Na2SO4), filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography (Hex/EtOAc, 1 :4) to afford 250 mg of the title compound as a purple solid: ES-MS: 314.0 / 316.0 [M+H]+; tR= 2.63 min (System 1 ); Rf = 0.09 (Hex/EtOAc, 1 :4).
References:
WO2009/141386,2009,A1 Location in patent:Page/Page column 121