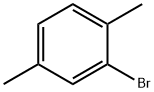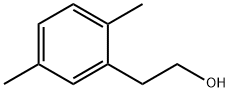
2,5-DIMETHYLPHENETHYL ALCOHOL synthesis
- Product Name:2,5-DIMETHYLPHENETHYL ALCOHOL
- CAS Number:6972-51-6
- Molecular formula:C10H14O
- Molecular Weight:150.22

50-00-0
868 suppliers
$10.00/25g
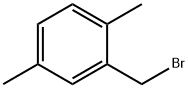
50837-53-1
31 suppliers
$60.00/100mg

6972-51-6
62 suppliers
$55.00/5G
Yield:6972-51-6 94.73%
Reaction Conditions:
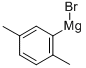
Stage #1: 2,5-dimethylbenzyl bromidewith iodine;magnesium in diethyl ether; for 2.16 h;Inert atmosphere;Reflux;
Stage #2: formaldehyd in diethyl ether at 20;Inert atmosphere;Temperature;
Steps:
1-3 Example 2:
In a 250mL dry four-necked flask, evacuate and replace the system three times with nitrogen, and then add 60mL of anhydrous ether in sequence under the protection of nitrogen. 3.30 g of magnesium dust (molecular weight 24.3, 135.80 mmol, 1.05 eq) and a small grain of iodine (50 mg), heated to 30 °C. 2,5-dimethyl benzyl bromide was slowly added dropwise approximately one-fifth of ether solution [25.75 g of 2,5-dimethylbromobenzyl(Molecular weight 199.09, 129.33 mmol, 1 eq) dissolved in 30 mL of ether]. No significant increase in temperature, stirred at this temperature for about 30 minutes at which time the temperature was observed to rise significantly, reaching reflux, the yellow solution, while faded, becomes gray. The remaining 2,5-dimethylbromobenzyl ether solution was slowly added dropwise, and the drop was completed in about 40 minutes. After dripping and holding for 1 hour, it was observed that the magnesium dust in the reaction solution had basically disappeared. The temperature of the reaction solution was reduced to 20 °C, and paraformaldehyde (molecular weight 30.03, 258.66 mmol, 2eq, a total of 7.77g, divided into 7 times, 1.11g at a time, 20 minutes each time) was added in batches, and kept at 20 °C after the addition. Stirring was continued for about 3 hours. 50 mL of saturated ammonium chloride solution was added to extract the reaction, and the mixture was extracted three times with 60 mL of ethyl acetate. The ethyl acetate phases were combined and the ethyl acetate was evaporated to dryness under negative pressure to obtain 18.82 g (molecular weight 150.22, theoretically 19.43 g) of a white solid. The purity determined by HPLC was 97.8%, which was 2,5-dimethylphenylethanol, and the mass yield was 94.73%.
References:
CN110305009,2019,A Location in patent:Paragraph 0037-0051
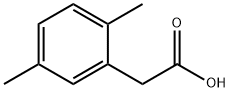
13612-34-5
240 suppliers
$5.00/1g

6972-51-6
62 suppliers
$55.00/5G
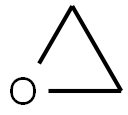
75-21-8
233 suppliers
$39.10/1mL
30897-86-0
54 suppliers
$130.00/10g

6972-51-6
62 suppliers
$55.00/5G