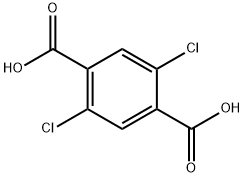
2,5-DICHLOROTEREPHTHALIC ACID synthesis
- Product Name:2,5-DICHLOROTEREPHTHALIC ACID
- CAS Number:13799-90-1
- Molecular formula:C8H4Cl2O4
- Molecular Weight:235.02

1124-05-6
195 suppliers
$6.00/5g

13799-90-1
154 suppliers
$5.00/250mg
Yield:13799-90-1 53%
Reaction Conditions:
Stage #1: 1,4-dichloro-2,5-dimethylbenzenewith pyridine;potassium permanganate in water at 100; for 12 h;
Stage #2: with hydrogenchloride in water; pH=1;
Steps:
4
Example 4Preparation of Nanopigment Red 122 (Method of Making II)Synthesis of dichloro terephthalic acid; In a 250 mL round bottom flask were introduced 5 g (0.028 mol) 2,5-dichloro-p-xylene, 26 g (0.165 mol) potassium permanganate, 80 mL pyridine and 20 mL deionized water. The mixture was heated to 100° C. with stirring for 12 hours. The brown manganese oxide was filtered while the suspension was still hot, and the brown solid reslurried twice with 100 mL deionized water. The liquids were combined and the solvents removed in vacuum. The yellowish syrupy liquid obtained was acidified with hydrochloric acid to a pH of 1. The white solid was filtered using a glass frit and dried in a vacuum oven at 50° C. overnight. Yield: 53-87%.Synthesis of 2,5-di-(p-toluidino)-terephthalic acids; In a 3 neck round bottom flask fitted with Argon inlet and magnetic stirring were charged; p-toluidine 23.19 g (0.216 mol), 2,5-dichloro-terephthalic acid 3.6 g (0.014 mol), anhydrous K2CO34.6 g (0.033 mol), anhydrous Copper (II) acetate 0.060 g (0.00033 mol), potassium iodide 0.750 g (0.0045 mol), ethylene glycol 16.8 g (0.271 mol) and deionized water 3.8 g (0.211 mol). The mixture was heated to 130° C. for 12 hours under argon. The reaction mixture was cooled to room temperature and diluted with 50 mL deionized water. Hydrochloric acid was added with stirring up to a pH of 1. The resultant dark solid is filtered using a glass frit. The solid was then dissolved into a solution of pH 7 containing ammonium hydroxide (3 mL) and deionized water (250 mL). The undissolved solid was filtered. The resultant yellow-green solution was acidified with acetic acid up to a pH of 3÷4. Upon acidification a dark brown solid formed. The solid was filtered using a glass frit and dried in a vacuum oven at 100° C. overnight. The resultant yield was 31%.Synthesis of the quinacridone PR122 nano; In a 250 mL round bottom flask fitted with a magnetic stirring bar were charged: 15 g polyphosphoric acid, 1 g powder of 2,5,di-(-toluidino)-terephthalic acids. The mixture was heated at 160° C. for two hours. A dark violet reddish color appeared. The reaction mixture was allowed to cool at room temperature and was diluted with 80 mL concentrated sulfuric acid. The resultant solution was transferred into a dropping funnel. The purple solution was added dropwise with stirring to a resin kettle containing 100 mL N-methyl-2-pyrrolidinone and 1.96 g (0.002 mol) SPAN 85. During the addition, the temperature was maintained below 45° C. When the addition finished, the mixture was stirred at room temperature for 30 minutes and filtered using a glass frit. The resulted solid was reslurried in 300 mL isopropanol, filtered on a glass frit and reslurried in 300 mL deionized water. After filtration on a glass frit the product was freeze dried for 48 hours. The resultant yield was 50%. (D50=100+/-1.4 nm, GSD=1.71+/-0.04) The particle morphology and range in size observed by Transmission Electron Microscopy showed regularly shaped particles consisting of thin, rounded platelets between about 50 and about 100 mm.
References:
US7427323,2008,B1 Location in patent:Page/Page column 21-22

1124-05-6
195 suppliers
$6.00/5g

13799-90-1
154 suppliers
$5.00/250mg
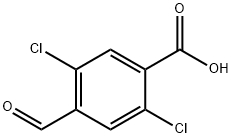
128230-05-7
2 suppliers
inquiry
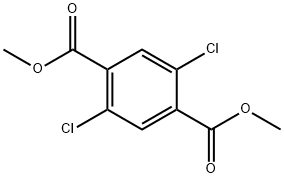
3293-89-8
19 suppliers
inquiry

13799-90-1
154 suppliers
$5.00/250mg

95-94-3
137 suppliers
$10.00/5g
124-38-9
127 suppliers
$214.00/14L

13799-90-1
154 suppliers
$5.00/250mg
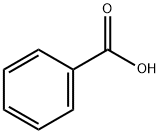
65-85-0
1355 suppliers
$10.00/25g
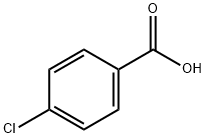
74-11-3
572 suppliers
$5.00/1g
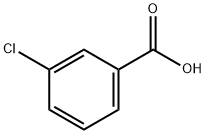
535-80-8
402 suppliers
$5.00/5g
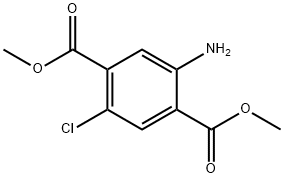
32888-87-2
10 suppliers
inquiry

13799-90-1
154 suppliers
$5.00/250mg
