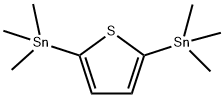
2,5‐ bis(triMethylstannyl)th iophene synthesis
- Product Name:2,5‐ bis(triMethylstannyl)th iophene
- CAS Number:86134-26-1
- Molecular formula:C10H20SSn2
- Molecular Weight:409.75
188290-36-0
1 suppliers
inquiry
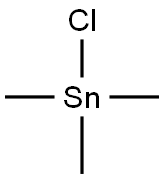
1066-45-1
148 suppliers
$26.03/2g

86134-26-1
67 suppliers
$25.00/100mg
Yield:86134-26-1 90%
Reaction Conditions:
Stage #1: thiophenewith n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 1.5 h;
Stage #2: trimethyltin(IV)chloride in tetrahydrofuran;hexane at 20; for 1 h;
Steps:
General procedure for synthesis of 3a-3f
General procedure: A solution of n-BuLi (2.20 mol/L in hexane, 1.00 mL, 2.20mmol, 2.20 equiv.) was added dropwise to a solution of 2a(68.1 mg, 1.00 mmol, 1.00 equiv.) in anhydrous tetrahydrofuran(THF, 15 mL) in a Schlenk flask at 78 °C. After 1 hat 78 °C, the reaction mixture was stirred at ambient temperature for 30 min. Then, Me3SnCl (1.00 mol/L in THF,2.50 mL, 2.50 mmol, 2.50 equiv.) was added and the stirringwas maintained for 1 h. After dilution with ethyl acetate(EtOAc, 50 mL), the mixture was washed with a saturatedaqueous solution of KF (20 mL) and then water, driedover MgSO4 and solvent was removed by distillation undervacuum. The residue was recrystallized from methanol togive 2,5-bis(trimethylstannyl)thiophene (3a) (369.8 mg,0.90 mmol, 90%) as a white crystal
References:
Zhang, Xin;Yao, Jiannian;Zhan, Chuanlang [Science China Chemistry,2016,vol. 59,# 2,p. 209 - 217]
3141-27-3
307 suppliers
$5.00/10g

1066-45-1
148 suppliers
$26.03/2g

86134-26-1
67 suppliers
$25.00/100mg
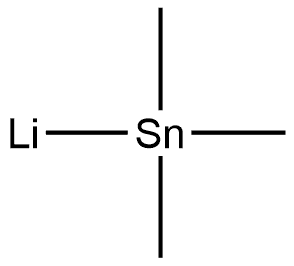
17946-71-3
1 suppliers
inquiry

3141-27-3
307 suppliers
$5.00/10g

86134-26-1
67 suppliers
$25.00/100mg