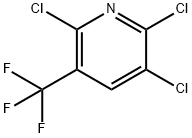
2,5,6-TRICHLORO-3-TRIFLUOROMETHYLPYRIDINE synthesis
- Product Name:2,5,6-TRICHLORO-3-TRIFLUOROMETHYLPYRIDINE
- CAS Number:80289-91-4
- Molecular formula:C6HCl3F3N
- Molecular Weight:250.43
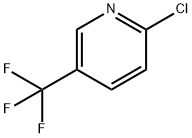
52334-81-3
429 suppliers
$5.00/1g

80289-91-4
37 suppliers
inquiry
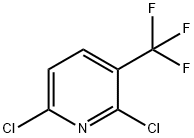
55304-75-1
146 suppliers
$12.00/1g

69045-84-7
399 suppliers
$10.00/5g
Yield:-
Reaction Conditions:
Stage #1: 2-chloro-5-trifluoromethylpyridine under 15001.5 Torr; for 2 h;Autoclave;Inert atmosphere;
Stage #2: with chlorine at 0 - 200; under 15001.5 Torr; for 20 h;Inert atmosphere;
Steps:
2 EXAMPLE 1
General procedure: 2-chloro-5-trifluoromethylpyridine (90.8 g, 0.5 mol) and 15% WCl6/AC (WCl6 supported on activated carbon, the load was 15 wt %, 12 g) were added into a 250 mL autoclave (Inconel alloy), after the kettle cover was installed, a nitrogen gas of 2 MPa was charged and the pressure was maintained for 2 h, a leakage detection was conducted to the reaction kettle, after the reaction kettle was confirmed to be gastight, it was placed into an ice ethanol bath for cooling, when the reaction kettle was cooled to 0° C., about 37.5 g of chlorine gas (0.5 mol) was charged into the reaction kettle from the reaction kettle gas phase tube, then the reaction kettle was placed into a heating jacket with a magnetic stirrer, under a stirring condition the reaction system was heated to 150° C., at this time the pressure of the reaction system was about 2.0 MPa, at this temperature continuously reacted for 20 h. After the reaction was finished, when the temperature of the reaction system dropped to room temperature, nitrogen gas was charged into the reaction kettle from a liquid phase tube and a replacement was conducted for 30 min (the tail gas being replaced out was introduced into a alkaline washing bottle for absorption, and neutralized), the reaction kettle was opened, the catalyst and the products were separated by filtration, and a 10 wt % of NaOH solution was added to the products for neutralization, extracted, and liquid separated, to obtain an oily product. The oily product obtained was dried on anhydrous sodium sulfate then weighed and the mass was 107.0 g, a qualitative analysis was conducted by GC-MS, and a quantitative analysis was conducted by gas chromatography internal standard method. The conversion of 2-chloro-5-trifluoromethylpyridine as well as the selectivity and the yield of the chlorination reaction product are seen in Table 1.
References:
US2020/102273,2020,A1 Location in patent:Paragraph 0147; 0161

55304-75-1
146 suppliers
$12.00/1g

80289-91-4
37 suppliers
inquiry