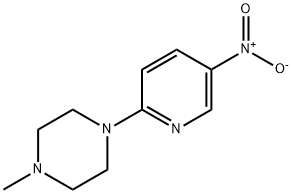
2-(4-Methylpiperazin-1-yl)-5-nitropyridine synthesis
- Product Name:2-(4-Methylpiperazin-1-yl)-5-nitropyridine
- CAS Number:55403-34-4
- Molecular formula:C10H14N4O2
- Molecular Weight:222.24
Yield:55403-34-4 99%
Reaction Conditions:
in acetonitrile; for 1.5 h;Heating / reflux;
Steps:
4
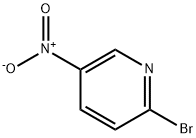
Intermediate 4l-Methyl-4-(5-nitro-2-pyridinyl)piperazineTo a solution of 2-bromo-5-nitropyridine (22.3 g, 110 mmol) in CH3CN (200 mL) was added JV-methylpiperazine (30.5 mL, 275 mmol) and the resulting mixture was heated with stirring to reflux. After 90 min, the reaction was cooled to room temperature and concentrated to dryness. The solids were partitioned between water and EtOAc. The organic layer was separated and washed with brine, dried (MgSO4), filtered and concentrated to dryness affording the title compound as a yellow solid (24.2 g, 99%). LC-MS (ES) m/z = 223 [M+H]+.
References:
WO2008/147831,2008,A1 Location in patent:Page/Page column 17