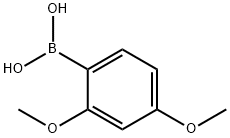
2,4-Dimethoxybenzeneboronic acid synthesis
- Product Name:2,4-Dimethoxybenzeneboronic acid
- CAS Number:133730-34-4
- Molecular formula:C8H11BO4
- Molecular Weight:181.98
5419-55-6
380 suppliers
$12.00/5g
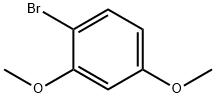
17715-69-4
298 suppliers
$9.00/10g

133730-34-4
302 suppliers
$6.00/1g
Yield:133730-34-4 610.2mg
Reaction Conditions:
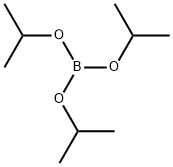
Stage #1: Triisopropyl borate;1-Bromo-2,4-dimethoxybenzenewith n-butyllithium in tetrahydrofuran;hexane;water at -78 - 40; for 2.66667 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;hexane;water;
Steps:
33.a a) 2,4-Dimethoxyphenylboronic acid
a)
2,4-Dimethoxyphenylboronic acid
576 μl of 1-bromo-2,4-dimethoxybenzene was dissolved in 5.8 ml of tetrahydrofuran and 3 ml of 1.6 mol/l solution of n-butyl lithium in hexane was added dropwise at -78° C. under argon atmosphere.
Then, 1.1 ml of triisopropylborate was added, and after stirred at -78° C. for 40 minutes, the mixture was stirred at room temperature for 2 hours.
To the reaction mixture, 40 ml of water and 1 ml of 5N-hydrochloric acid was added, and extracted with 50 ml of ethyl acetate.
After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure, and purified by silica gel column chromatography (hexane:ethyl acetate=2:1) to yield 610.2 mg of the title compound.
1H-NMR (CDCl3); δ (ppm) 3.85 (3H, s), 3.89 (3H, s), 5.81 (2H, s), 6.46 (1H, s), 6.56 (1H, dd, J=2.0, 8.4 Hz), 7.77 (1H, d, J=8.4 Hz).
References:
US2013/317074,2013,A1 Location in patent:Paragraph 0482; 0483; 0484

17715-69-4
298 suppliers
$9.00/10g

133730-34-4
302 suppliers
$6.00/1g

67-56-1
756 suppliers
$9.00/25ml
55124-35-1
23 suppliers
$60.00/100mg

17715-69-4
298 suppliers
$9.00/10g

133730-34-4
302 suppliers
$6.00/1g
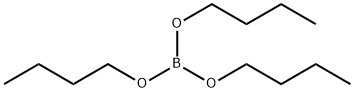
688-74-4
327 suppliers
$10.40/25mL
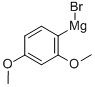
138109-49-6
32 suppliers
$226.00/50mL

133730-34-4
302 suppliers
$6.00/1g
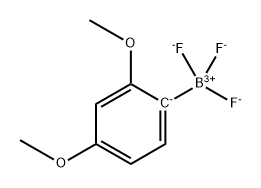
1033691-64-3
0 suppliers
inquiry

133730-34-4
302 suppliers
$6.00/1g