
2-(3-METHOXYPHENYL)PYRROLIDINE synthesis
- Product Name:2-(3-METHOXYPHENYL)PYRROLIDINE
- CAS Number:103861-77-4
- Molecular formula:C11H15NO
- Molecular Weight:177.24
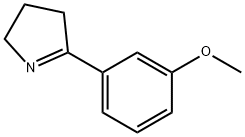
71291-54-8
1 suppliers
inquiry

103861-77-4
76 suppliers
$42.00/100mg
Yield: 99%
Reaction Conditions:
Stage #1:2-(3-anisyl)-Δ1-pyrroline with sodium tetrahydroborate in ethanol at 20;
Stage #2: with hydrogenchloride;water in ethanol at 0 - 20; for 0.75 h;
Stage #3: with sodium hydroxide;water in ethanol
Steps:
39.2
Step 2. 2-(3-Methoxy-phenyl)-pyrrolidine. A solution of 5-(3-methoxy-phenyl)-3,4-dihydro-2H-pyrrole (0.21 mol) in absolute ethanol (1.2 M) was treated portionwise with NaBH4 (1.0 equiv.). The resultant mixture was stirred at rt overnight. The mixture was cooled to 0° C. and slowly quenched with 1 N HCl. The mixture was acidified to a pH of 1 with 3 N HCl and was stirred at rt for 45 min. The resulting mixture was again cooled to 0° C., and was treated with 1 N NaOH until basic. The aqueous mixture was extracted with CH2Cl2 (×3). The combined extracts were washed with brine, dried (MgSO4), filtered and concentrated to give the crude product. Chromatography (EtOAc/hexanes) gave 37.0 g (99%) of the desired product. MS: exact mass calcd for C11H15NO, 177.1; m/z found, 178.1 [M+H]+. 1H NMR (DMSO-d6): 7.23 (m, 1H), 7.05 (m, 1H), 6.98 (d, J=7.6, 1H), 6.79 (dd, J=2.8, 8.0, 1H), 4.20 (m, 1H), 3.74 (s, 3H), 3.15 (m, 1H), 3.03 (m, 1H), 2.19 (m, 1H), 1.86 (m, 2H), 1.69 (m, 1H).
References:
Apodaca, Richard;Barbier, Ann J.;Carruthers, Nicholas I.;Gomez, Leslie A.;Keith, John M.;Lovenberg, Timothy W.;Wolin, Ronald L. US2006/293316, 2006, A1 Location in patent:Page/Page column 41
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

103861-77-4
76 suppliers
$42.00/100mg
![Tert-butyl N-[4-(3-methoxyphenyl)-4-oxobutyl]carbamate](/CAS/20210305/GIF/914954-34-0.gif)
914954-34-0
1 suppliers
inquiry

103861-77-4
76 suppliers
$42.00/100mg
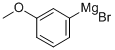
36282-40-3
96 suppliers
$88.40/100ml

103861-77-4
76 suppliers
$42.00/100mg
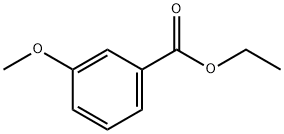
10259-22-0
80 suppliers
$15.00/5g

103861-77-4
76 suppliers
$42.00/100mg