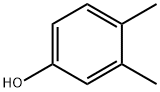
2,3-dimethylphenol synthesis
- Product Name:2,3-dimethylphenol
- CAS Number:526-75-0
- Molecular formula:C8H10O
- Molecular Weight:122.16
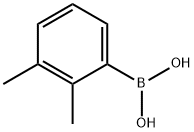
183158-34-1
231 suppliers
$7.00/1g

526-75-0
204 suppliers
$10.00/1g
Yield:526-75-0 96%
Reaction Conditions:
with dihydrogen peroxide in acetonitrile at 30 - 35; for 0.166667 h;Schlenk technique;
Steps:
General procedure for the oxidation of arylboronic acids to phenols (Table 5)
General procedure: an oven-dried Schlenk flask was allowed to cool to room temperature and charged sequentially with arylboronic acid (1 mmol), MeCN (3.0 mL) and silica chloride (0.5 mmol). The reaction was then activated by addition of 30% H2O2 (1.0 equiv.) and stirred at 30-35 °C for the required time as given in Table 5. The progress of reaction was monitored by TLC. After complete conversion of starting material, the reaction mixture was filtered to remove silica gel. The reaction mixture was neutralized with 5% NaHCO3 solution (5 mL). Then the product was extracted with ethyl acetate (30 mL) and subsequently washed with distilled water (10 mL). The organic extract was dried over Na2SO4 and the solvent was removed under reduced pressure. The resultant product was purified by column chromatography using silica gel with n-hexane and ethyl acetate as solvent to get the pure product. The structure of the product was confirmed by GC-MS, M.P. and 1H NMR spectroscopic techniques.
References:
Wagh, Ravindra B.;Nagarkar, Jayashree M. [Tetrahedron Letters,2017,vol. 58,# 33,p. 3323 - 3326] Location in patent:supporting information
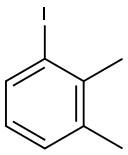
31599-60-7
131 suppliers
$6.00/1g

526-75-0
204 suppliers
$10.00/1g

90-05-1
742 suppliers
$5.00/1g
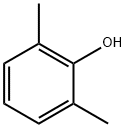
576-26-1
335 suppliers
$10.00/25g

526-75-0
204 suppliers
$10.00/1g

108-39-4
479 suppliers
$13.00/25g
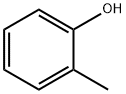
95-48-7
438 suppliers
$18.00/25g
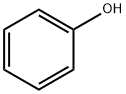
108-95-2
734 suppliers
$14.00/25g