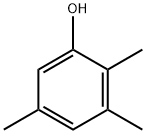
2,3,5-Trimethylphenol synthesis
- Product Name:2,3,5-Trimethylphenol
- CAS Number:697-82-5
- Molecular formula:C9H12O
- Molecular Weight:136.19
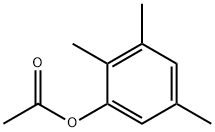
34649-27-9
3 suppliers
inquiry

697-82-5
369 suppliers
$7.00/10g
Yield:-
Reaction Conditions:
with FAU zeolite CBV 720 in toluene at 149.84; for 6 h;Inert atmosphere;Reagent/catalyst;
Steps:
General procedure for the following rearrangement reactions
General procedure: The following rearrangement reactions were all carried out in an identical manner:The rearrangement was carried out in pressure tube reactors (V = 21 cm3, Ace Glass) equipped with a magnetic stirrer and oil bath. The pre-dried catalyst (340 mg, 573 K, 0.1 mbar, 12 h), and substrate (2.5 mmol) in 10 cm3anhydrous toluene were added into the reactor. After replacing air with Ar three times (PanGas, purity 5.0), the reactor was heated to T = 423 K at autogenous pressure for 6 h. The reaction mixture was quenched with ice-water, and 0.01 cm3of the reaction mixture was added into 1 cm3acetonitrile (99.9%, AcroSeal) for further analysis. Samples were analyzed using an Agilent 1260 Infinity HPLC equipped with an Agilent Zorbax C18 column and both DAD and RID detectors. The concentrations of substrates and products were calibrated with reference to the respective pure standards. The conversion (X,) and the product selectivity (S,) were calculated according to equations (1) and (2), respectively. Equation (1) Equation (2) where n°sand n are the moles of substrate before and after the reaction, respectively, while n) is the mole of product i. The yield is calculated as the product of conversion and selectivity of the desired product.
References:
WO2021/156214,2021,A1 Location in patent:Page/Page column 9-10; 14

95-63-6
279 suppliers
$15.69/250mg
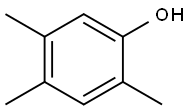
496-78-6
57 suppliers
$69.00/250mg

697-82-5
369 suppliers
$7.00/10g
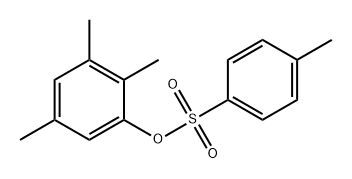
312609-91-9
0 suppliers
inquiry

697-82-5
369 suppliers
$7.00/10g
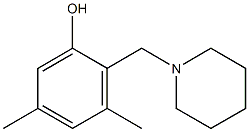
68097-90-5
0 suppliers
inquiry

697-82-5
369 suppliers
$7.00/10g
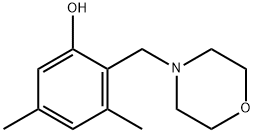
14944-83-3
8 suppliers
inquiry

697-82-5
369 suppliers
$7.00/10g