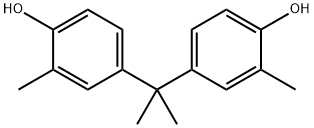
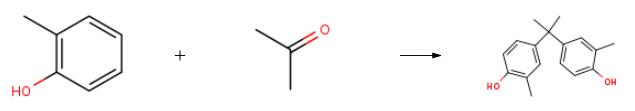
2,2-Bis(4-hydroxy-3-methylphenyl)propane synthesis
- Product Name:2,2-Bis(4-hydroxy-3-methylphenyl)propane
- CAS Number:79-97-0
- Molecular formula:C17H20O2
- Molecular Weight:256.34
Yield:79-97-0 91%
Reaction Conditions:
with methanol;sulfuric acid;3-mercaptopropionic acid in toluene at 40; for 2.5 h;Inert atmosphere;Temperature;Reagent/catalyst;Solvent;
Steps:
9-23; 40 example 19
General procedure: In a full-jacket type 1 L separable flask equipped with a thermometer, a stirrer, and a 100 mL dropping funnel, 26.1 g (0.4 mol) of isopropyl alcohol was added under a nitrogen atmosphere, and then 58.3 g (0.5 mol) of 90% by weight sulfuric acid was slowly added thereto. Thereafter, 54.5 g of toluene, 191.5 g (1.8 mol) of ortho-cresol, and 5.5 g (0.03 mol) of dodecanethiol were added thereto, followed by setting the temperature in the separable flask to 40° C. In the dropping funnel, 42.5 g (0.7 mol) of acetone was placed, and it was slowly fed dropwise to the separable flask for 30 minutes. After completion of the dropwise addition of acetone, the reaction was allowed to proceed at 40° C. for 2 hours. After completion of the reaction, 100.0 g of toluene and 100.0 g of demineralized water were fed, and the temperature was increased to 80° C. After the temperature reached 80° C., the reaction liquid was left to stand to confirm that the precipitates generated during the reaction were dissolved into the organic phase and the aqueous phase. This was followed by extraction of the aqueous phase in the lower layer. Thereafter, saturated sodium hydrogen carbonate solution was added to the obtained organic phase to allow neutralization, followed by confirming that the pH of the aqueous phase in the lower layer, became not less than 9. After extraction of the aqueous phase in the lower layer, demineralized water was added to the obtained organic phase, and the resulting mixture was stirred for 10 minutes. Thereafter, the mixture was left to stand, and the aqueous phase was extracted. Part of the obtained organic phase was removed, and subjected to high-performance liquid chromatography to analyze the amount of bisphenol C produced. As a result, the reaction yield in terms of acetone was found to be 84 mol %.
References:
US2020/190004,2020,A1 Location in patent:Paragraph 0255-0279; 0296
95-48-7
438 suppliers
$18.00/25g
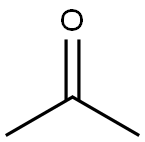
67-64-1
6 suppliers
$19.10/10ml

79-97-0
165 suppliers
$30.00/25g
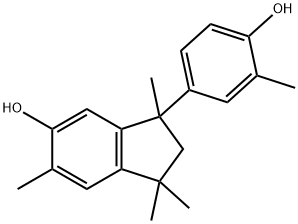
17689-00-8
0 suppliers
inquiry

95-48-7
438 suppliers
$18.00/25g

79-97-0
165 suppliers
$30.00/25g
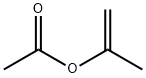
108-22-5
253 suppliers
$20.00/25mL

95-48-7
438 suppliers
$18.00/25g

79-97-0
165 suppliers
$30.00/25g