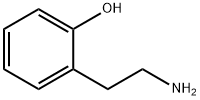
2-(2-aminoethyl)phenol synthesis
- Product Name:2-(2-aminoethyl)phenol
- CAS Number:2039-66-9
- Molecular formula:C8H11NO
- Molecular Weight:137.18
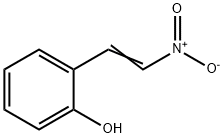
3156-43-2
15 suppliers
$60.00/5mg

2039-66-9
102 suppliers
$29.00/100mg
Yield:2039-66-9 65%
Reaction Conditions:
Stage #1: 2-(2-nitro-vinyl)-phenolwith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Stage #2: with water in tetrahydrofuran at 0;Inert atmosphere;
Steps:
4.2.1. 2-(2-Aminoethyl)phenol (3a)24
A solution of 2-(2-nitrovinyl)phenol 2a (860 mg, 5.21 mmol) in THF (12 mL) was added dropwise (over 1 h) to a stirred and cooled (0 °C) suspension of LiAlH4 (479 mg, 17.18 mmol) in THF (6 mL). Stirring was continued for 2 h at room temperature. The reaction mixture was cooled to 0 °C and then water was added. The organic solvent was removed in vacuo. The residue was dissolved in HCl (10%, 40 mL) and washed twice with EtOAc (20 mL). The combined organic layers were extracted twice with HCl (10%, 20 mL). The combined aqueous layers were treated with tartaric acid (4 equiv) and the pH was adjusted to >10 with concd NH3 and the aqueous layers were extracted three times with chloroform (30 mL). The combined organic layers were washed with water and brine, dried over anhydrous Na2SO4, and evaporated. The residue was purified by chromatography on silica gel using 3-10% 2 M NH3 in MeOH in CH2Cl2 to yield 3a as a white solid (464 mg, 3.38 mmol, 65%). 1H NMR (500 MHz, CDCl3): δ 7.12 (ddd, J=8.0, 8.0, 1.5 Hz, 1H), 6.99 (dd, J=7.5, 1.5 Hz, 1H), 6.90 (dd, J=8.0, 1.0 Hz, 1H), 6.76 (ddd, J=7.5, 7.5, 1.0 Hz, 1H), 5.40 (br, 1H), 3.09-3.07 (m, 2H), 2.80-2.78 (m, 2H).
References:
Hellal, Malik;Singh, Shambhavi;Cuny, Gregory D. [Tetrahedron,2012,vol. 68,# 6,p. 1674 - 1681] Location in patent:experimental part
2045-79-6
148 suppliers
$20.16/5ML

2039-66-9
102 suppliers
$29.00/100mg
14714-50-2
60 suppliers
inquiry

2039-66-9
102 suppliers
$29.00/100mg
![7-Oxabicyclo[4.1.0]hepta-2,4-diene-1-ethanamine](/CAS/20210305/GIF/73971-96-7.gif)
73971-96-7
0 suppliers
inquiry

2039-66-9
102 suppliers
$29.00/100mg
![7-Oxabicyclo[4.1.0]hepta-2,4-diene-2-ethanamine](/CAS/20210305/GIF/72251-59-3.gif)
72251-59-3
0 suppliers
inquiry

2039-66-9
102 suppliers
$29.00/100mg