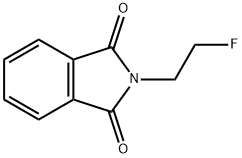
1H-Isoindole-1,3(2H)-dione, 2-(2-fluoroethyl)- synthesis
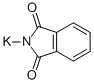
- Product Name:1H-Isoindole-1,3(2H)-dione, 2-(2-fluoroethyl)-
- CAS Number:442-31-9
- Molecular formula:C10H8FNO2
- Molecular Weight:193.17
3891-07-4
323 suppliers
$6.00/10g

442-31-9
5 suppliers
inquiry
Yield:442-31-9 97%
Reaction Conditions:
with TFEDMA;titanium tetrachloride in tetrahydrofuran at 20 - 45; for 2 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
1.1-1.3; 2; 1 Example 2
General procedure: Weigh 193g (1mol) of commercially available N-hydroxyethyl phthalimide with a purity of 99%, add it to a 1L three-necked flask with mechanical stirring, constant pressure feeding funnel and thermometer catheter, and add 100ml under nitrogen protection (About 80g) Dry acetonitrile. Turn on the water bath to heat, and add dropwise a mixed solution consisting of 375g (1.5mol) purified fluorinating reagent CHFClCF2NEt2 with a GC content of 95%, 5g boron trifluoride ether solution with a content of 46%, and 100ml of acetonitrile under stirring. During the dripping period, the internal temperature was maintained at 20-25. After the dripping, the reaction was continued for 1h, and then the temperature was raised to 45 to continue the reaction for 1h. During this time, the TLC dot plate was used to monitor the progress of the reaction. If the raw materials disappeared, the reaction was stopped and N- Fluorinated alkyl phthalimide fluorine-containing primary amine intermediate.Post-treatment of fluorine-containing primary amine intermediate product: Separate and remove the solvent acetonitrile under reduced pressure at 20°C and 40mmHg, then add 40°C absolute ethanol to dissolve the amide according to 60% of the mass of the remaining materials in the system and filter, and filter the residue at 15°C Dissolve and wash 4 times in anhydrous ethanol. Finally, the filter residue is dried in a blast oven at 80°C to a constant weight to obtain 183 g of N-fluoroethyl phthalimide with a purity of 99.5% and a yield of 94.3%.
References:
CN113045477,2021,A Location in patent:Paragraph 0030-0031; 0032-0034; 0058
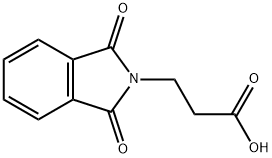
3339-73-9
144 suppliers
$10.00/1g

442-31-9
5 suppliers
inquiry