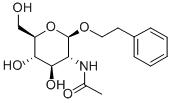
PHENYLETHYL 2-ACETAMIDO-2-DEOXY-BETA-D-GLUCOPYRANOSIDE synthesis
- Product Name:PHENYLETHYL 2-ACETAMIDO-2-DEOXY-BETA-D-GLUCOPYRANOSIDE
- CAS Number:197574-94-0
- Molecular formula:C16H23NO6
- Molecular Weight:325.36
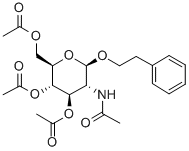
197574-92-8
20 suppliers
inquiry

197574-94-0
27 suppliers
inquiry
Yield:197574-94-0 95%
Reaction Conditions:
Stage #1: Acetic acid (2R,3S,4R,5R,6R)-3-acetoxy-2-acetoxymethyl-5-acetylamino-6-phenethyloxy-tetrahydro-pyran-4-yl esterwith sodium methylate in methanol at 20; for 2 h;
Stage #2: in methanol at 20; pH=7;
Steps:
4.1.1. General procedure for preparation of compounds 4a-k
General procedure: Compound 2 (2.1 g, 5.7 mmol) and substituted aromatic alcohol (3.8 mmol) were added to a suspension of zinc chloride (0.51 g, 3.8 mmol) and 4,4'-dimethoxytrityl chloride (1.28 g, 3.8 mmol) in dry dichloromethane (15 ml), then stirred overnight at room temperature. After diluting with dichloromethane, the mixture was washed with saturated aqueous sodium hydrogen carbonate. The solvent was removed in vacuum and the residue was purified by column chromatography (petroleum ether-EtOAc 2:1, v/v) to yield the glycosides 3a-k (54-71%). To compounds 3a-k (3.8 mmol) in MeOH (15 ml) was added NaOMe (0.2 g, 3.8 mmol) and the mixture was allowed to stir for 2 h at room temperature. On completion of the reaction as indicated by TLC (methanol/chloroform 1/5-1/3, v/v), the solution was neutralized with Amberlite IRA-120 (H+) resin to pH 7, then filtered and evaporated to dryness under reduced pressure afford 4a-k.
References:
Meng, Ying;Guo, Yibing;Ling, Yong;Zhao, Yahong;Zhang, Qi;Zhou, Xinyang;Ding, Fei;Yang, Yumin [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 18,p. 5577 - 5584] Location in patent:experimental part
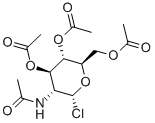
3068-34-6
136 suppliers
$44.79/1G

197574-94-0
27 suppliers
inquiry