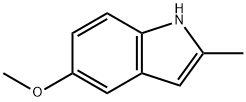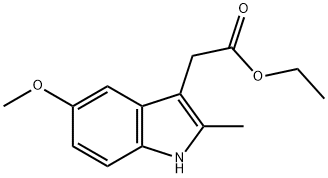
(5-METHOXY-2-METHYL-1H-INDOL-3-YL)-ACETIC ACID ETHYL ESTER synthesis
- Product Name:(5-METHOXY-2-METHYL-1H-INDOL-3-YL)-ACETIC ACID ETHYL ESTER
- CAS Number:17536-38-8
- Molecular formula:C14H17NO3
- Molecular Weight:247.29
19501-58-7
378 suppliers
$7.00/250mg
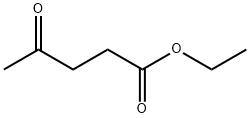
539-88-8
351 suppliers
$5.00/5g

17536-38-8
23 suppliers
inquiry
Yield:17536-38-8 76%
Reaction Conditions:
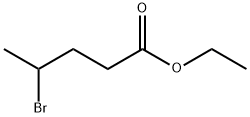
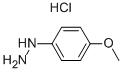
Stage #1: 4-methoxyphenylhydrazine hydrochloride;4-oxopentanoic acid ethyl esterwith sodium acetate;acetic acid for 3 h;Heating / reflux;
Stage #2: with hydrogenchloride;ethanol in 1,4-dioxane; for 15 h;Heating / reflux;
Steps:
24.24a
24a. Ethyl 2-(5-methoxy-2-methylindol-3-yl)acetateA stirred mixture of 4-methoxyphenylhydraζine hydrochloride (26 g, 0.149 mol), ethyl levulinate (21.5 g, 0.149 mol), and sodium acetate (12.2 g, 0.149 mol) in glacial acetic acid (200 mL) was heated at reflux for 3 hours. The reaction mixture was concentrated to dryness. The residue was dissolved in ethanol (120 mL), treated with 4M HCl in 1,4-dioxane (80 mL), and heated at a gentle reflux for 15 hours. The reaction mixture was concentrated to remove the volatiles, and the residue was taken up with EtOAc, washed with water, aqueous K2CO3, and brine. The organic layer was dried over Na2SO4, filtered, and concentrated. Chromatography of the residue (1:1 EtOAc:Hexane, silica gel) gave the title compound (28 g, 76% yield) as a viscous oil. 1H NMR (300 MHz, CDCl3) 57.83 (br s, IH), 7.06 (d, J= 8.7 Hz, IH), 7.00 (d, J = 2.4 Hz, IH), 6.77-6.73 (m, IH), 4.12 (q, J= 7.1 Hz, 2H), 3.84 (s, 3H), 3.64 (m, 2H), 2.32 (s, 3H), 1.23 (t, J= 7.1 Hz, 3H); Mass Spectrum (API-TIS) mJz 248 (MH+)
References:
WO2006/99416,2006,A1 Location in patent:Page/Page column 98

19501-58-7
378 suppliers
$7.00/250mg
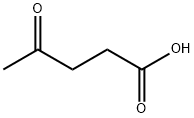
123-76-2
472 suppliers
$5.00/25g

17536-38-8
23 suppliers
inquiry

64-17-5
717 suppliers
$10.00/50g
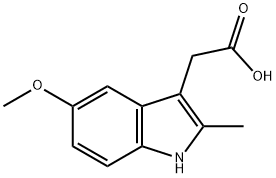
2882-15-7
154 suppliers
$12.00/100mg

17536-38-8
23 suppliers
inquiry